Deposition Date
2023-11-19
Release Date
2024-03-27
Last Version Date
2025-07-09
Entry Detail
PDB ID:
8R60
Keywords:
Title:
1918 H1N1 Viral polymerase heterotrimer in complex with 4 repeat serine-5 phosphorylated PolII peptide
Biological Source:
Source Organism(s):
Influenza A virus (A/Brevig Mission/1/1918(H1N1)) (Taxon ID: 88776)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
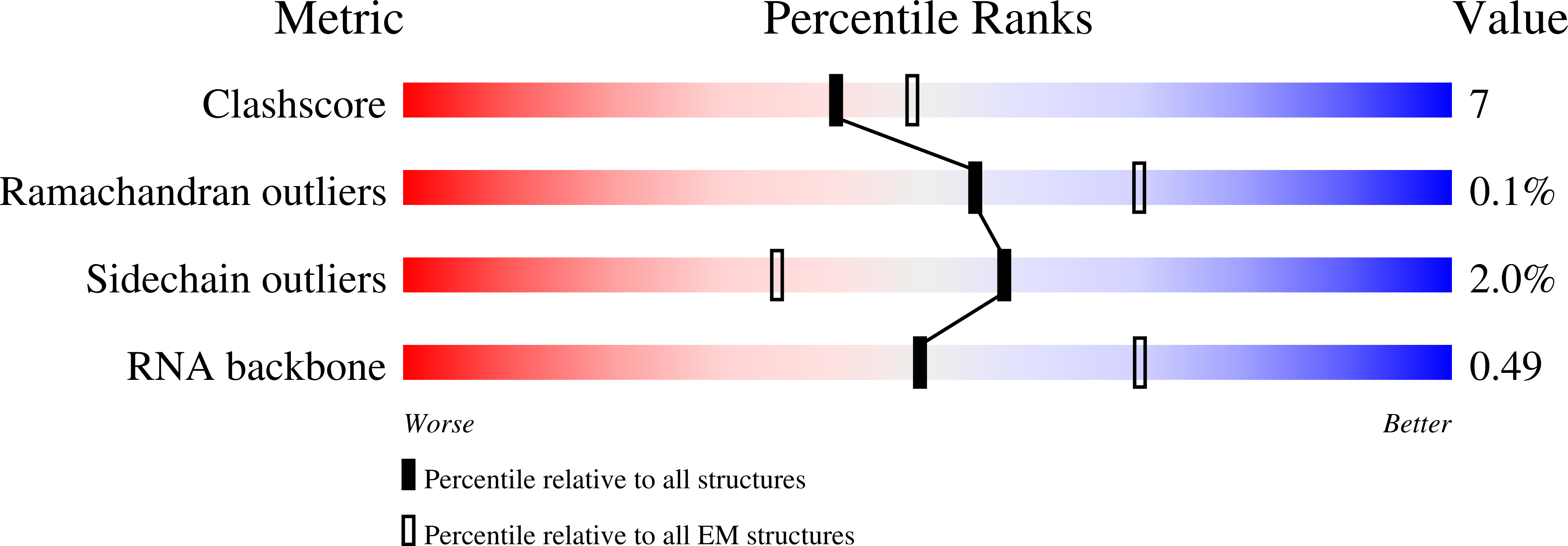
Experimental Method:
Resolution:
3.23 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE