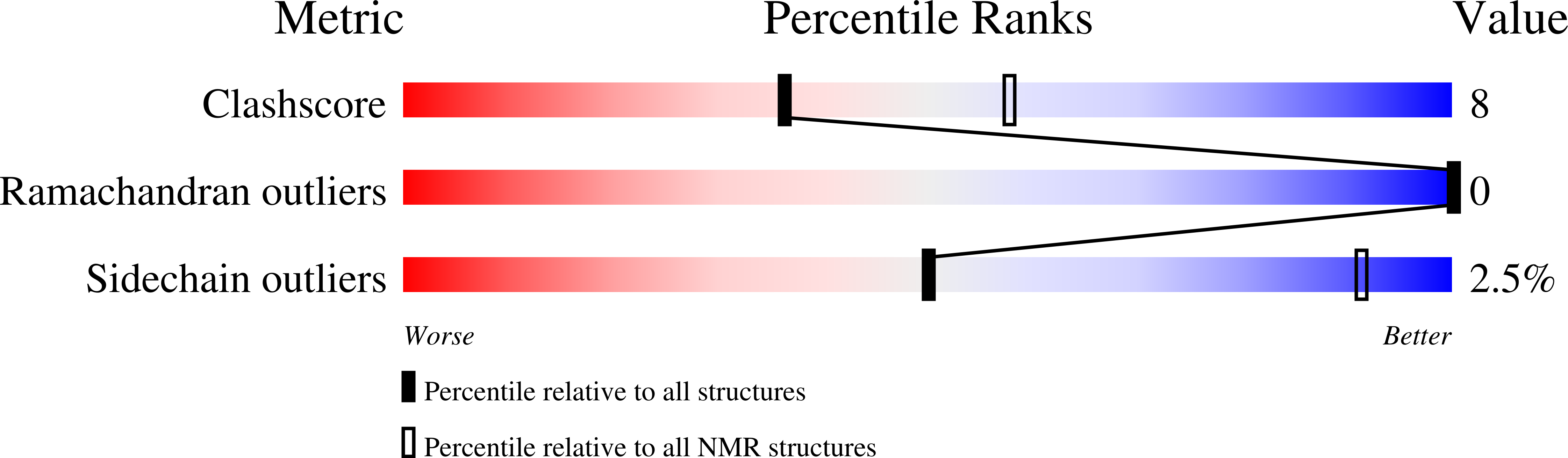
Deposition Date
2023-10-09
Release Date
2024-06-26
Last Version Date
2024-07-03
Entry Detail
PDB ID:
8QRX
Keywords:
Title:
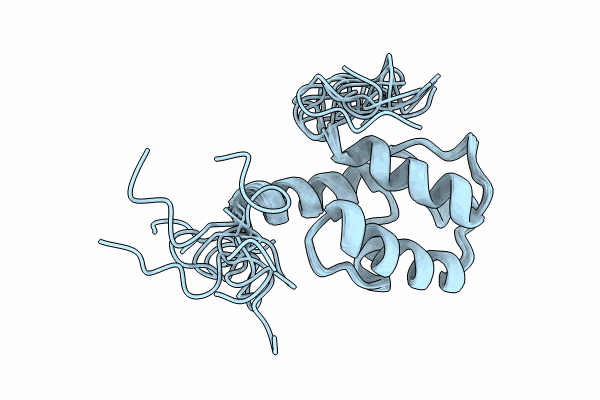
Solution NMR structure of the peptidyl carrier domain TomAPCP from the Tomaymycin non-ribosomal peptide synthetase in its substrate-loaded state
Biological Source:
Source Organism(s):
Streptomyces regensis (Taxon ID: 68263)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
100
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy