Deposition Date
2023-09-25
Release Date
2024-10-02
Last Version Date
2025-01-15
Entry Detail
PDB ID:
8QMW
Keywords:
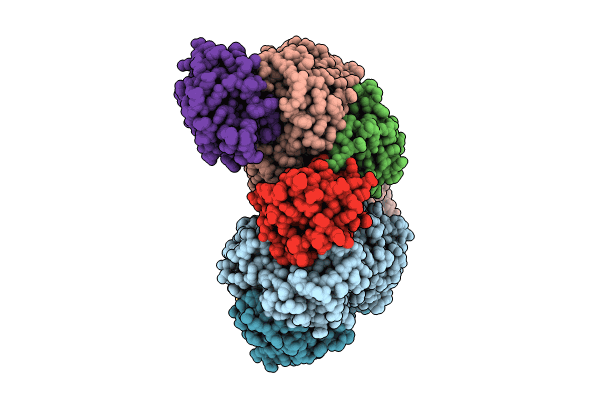
Title:
Non-obligately L8S8-complex forming RubisCO derived from ancestral sequence reconstruction and rational engineering in L8S8 complex with substitutions R269W, E271R, L273N
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
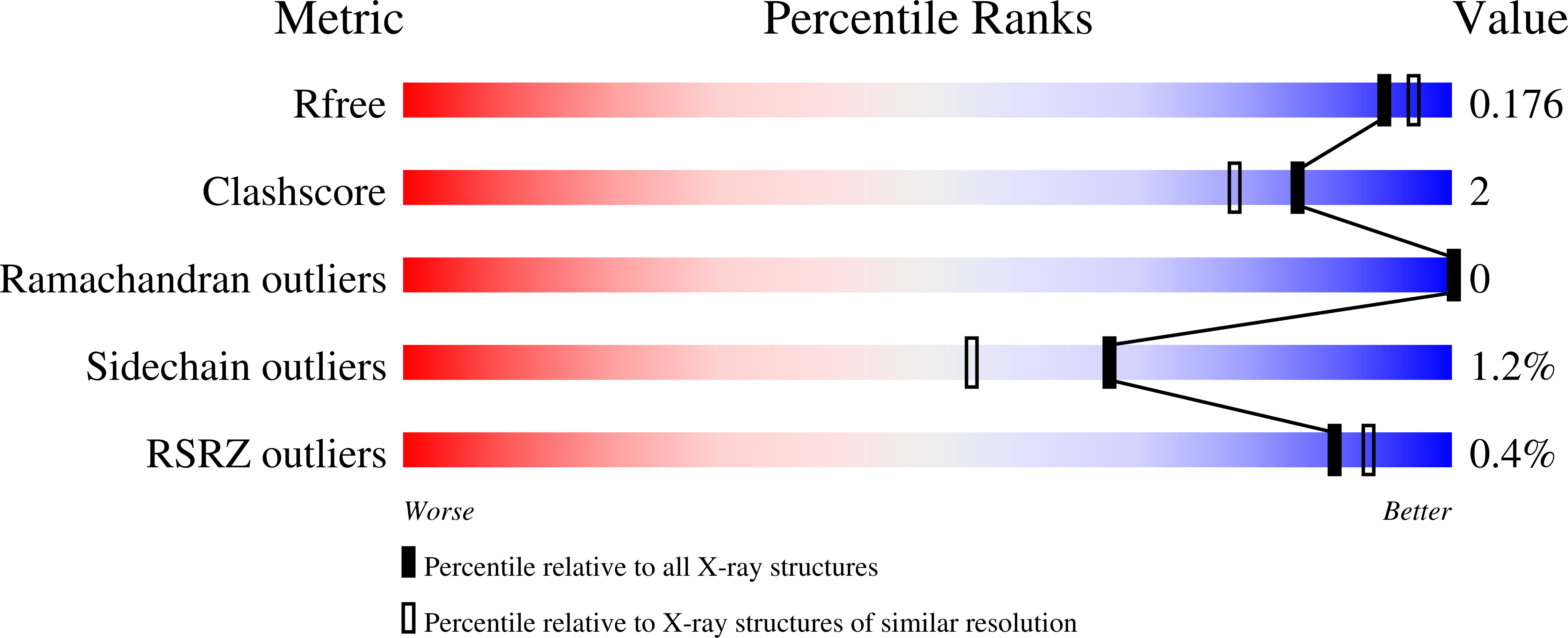
Resolution:
1.75 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
C 1 2 1