Abstact
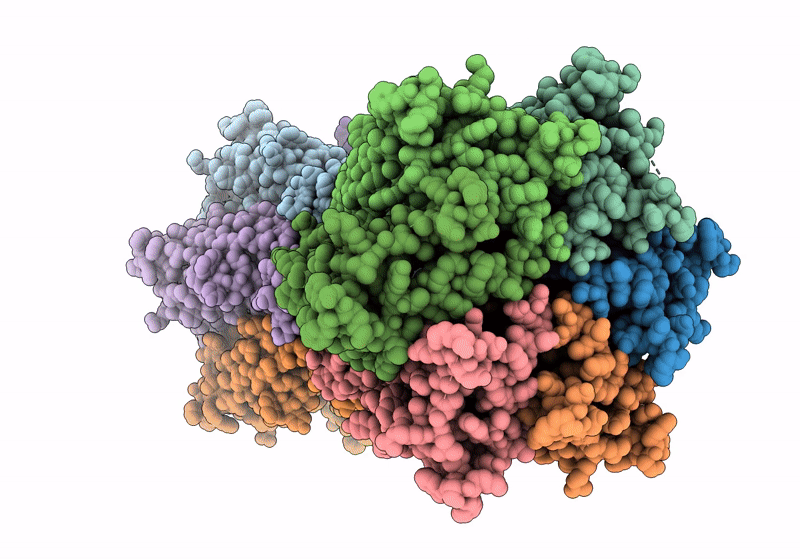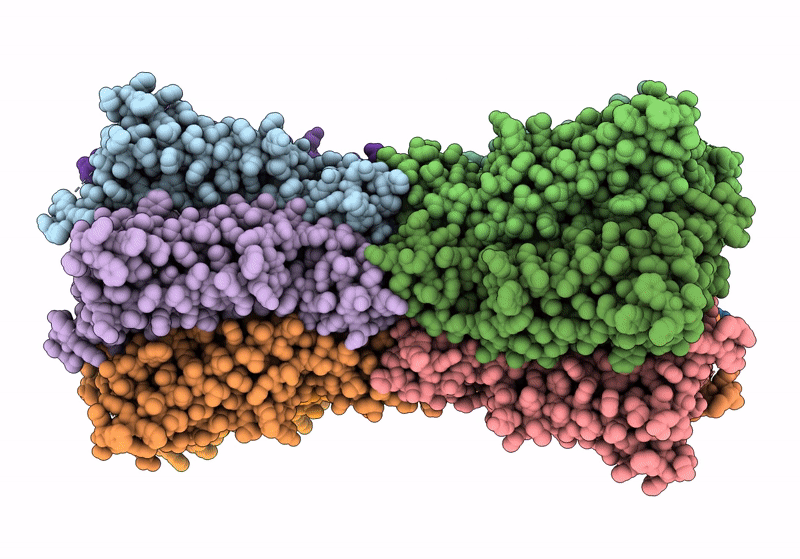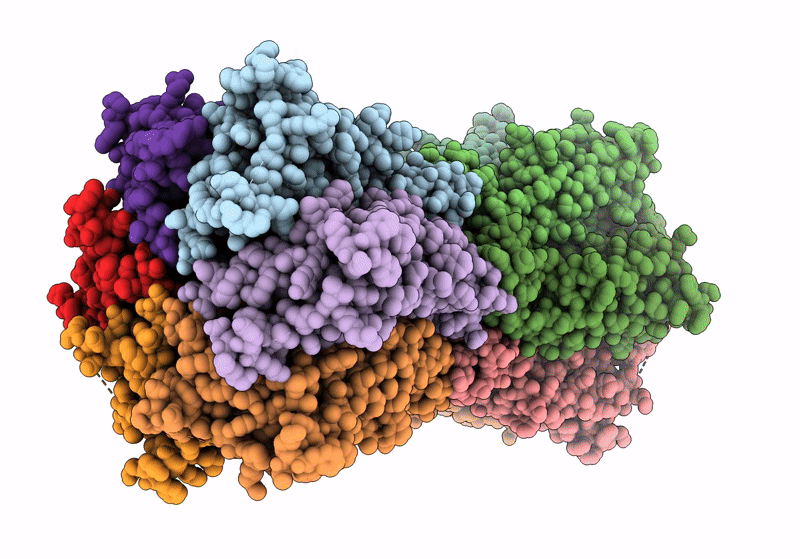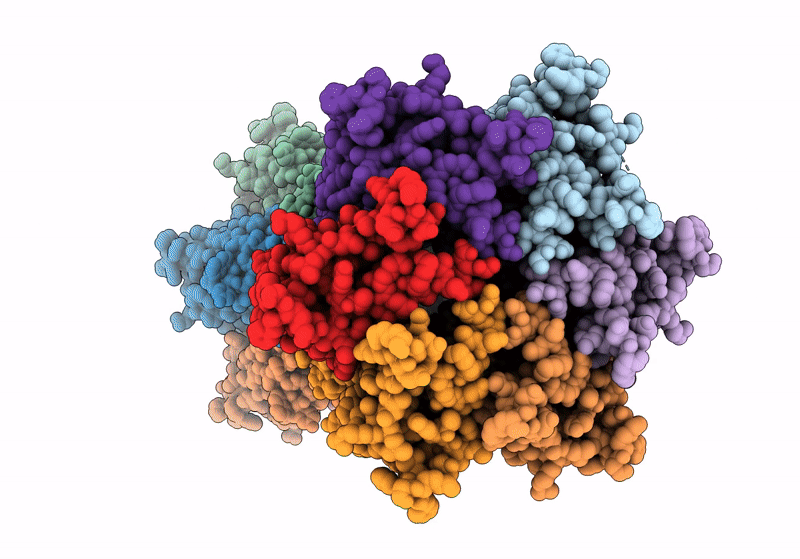
Gap junction intercellular communication (GJIC) between two adjacent cells involves direct exchange of cytosolic ions and small molecules via connexin gap junction channels (GJCs). Connexin GJCs have emerged as drug targets, with small molecule connexin inhibitors considered a viable therapeutic strategy in several diseases. The molecular mechanisms of GJC inhibition by known small molecule connexin inhibitors remain unknown, preventing the development of more potent and connexin-specific therapeutics. Here we show that two GJC inhibitors, mefloquine (MFQ) and 2-aminoethoxydiphenyl borate (2APB) bind to Cx32 and block dye permeation across Cx32 hemichannels (HCs) and GJCs. Cryo-EM analysis shows that 2APB binds to "site A", close to the N-terminal gating helix of Cx32 GJC, restricting the entrance to the channel pore. In contrast, MFQ binds to a distinct "site M", deeply buried within the pore. MFQ binding to this site modifies the electrostatic properties of Cx32 pore. Mutagenesis of V37, a key residue located in the site M, renders Cx32 HCs and GJCs insensitive to MFQ-mediated inhibition. Moreover, our cryo-EM analysis, mutagenesis and activity assays show that MFQ targets the M site in Cx43 GJC similarly to Cx32. Taken together, our results point to a conserved inhibitor binding site in connexin channels, opening a new route for development of specific drugs targeting connexins.