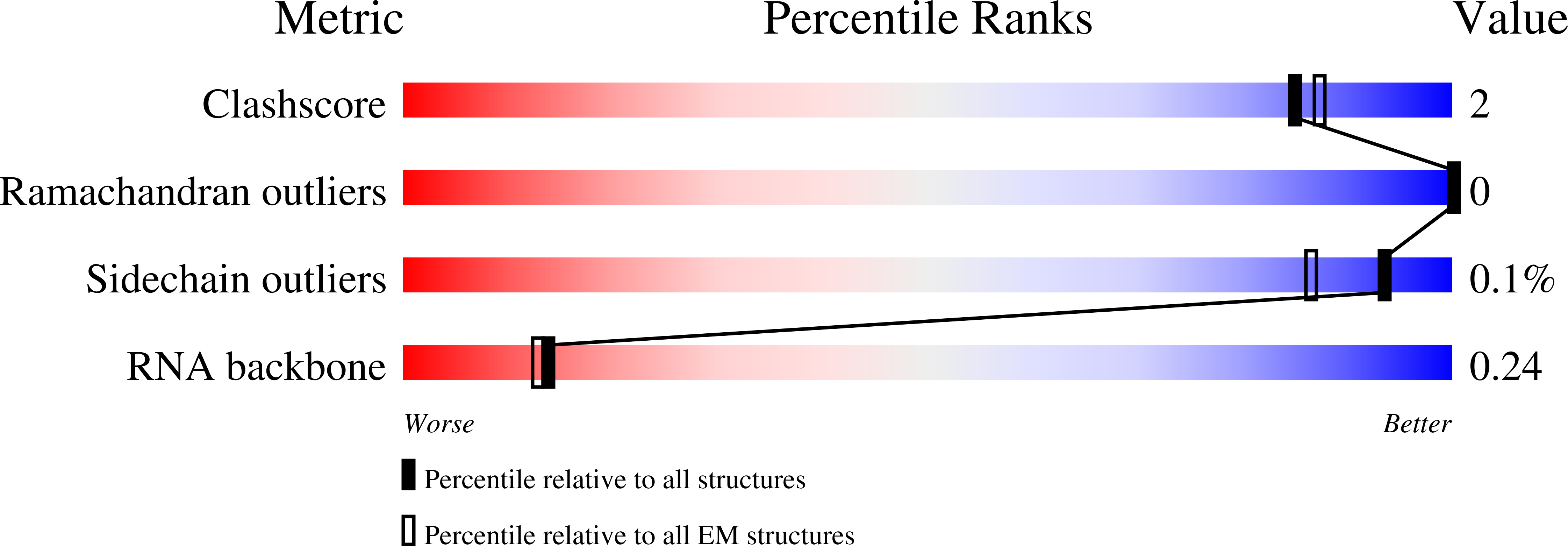
Deposition Date
2023-08-25
Release Date
2024-01-10
Last Version Date
2025-07-09
Entry Detail
PDB ID:
8QCF
Keywords:
Title:
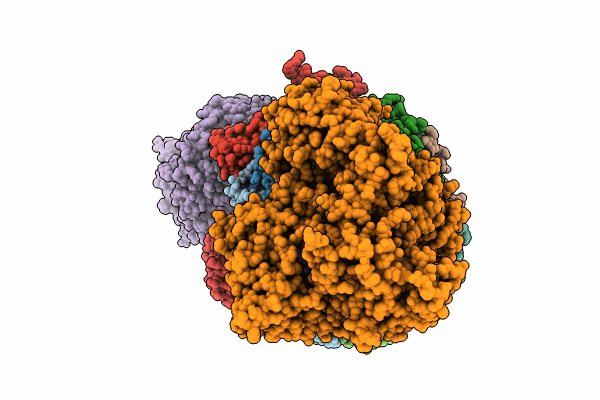
yeast cytoplasmic exosome-Ski2 complex degrading a RNA substrate
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.55 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE