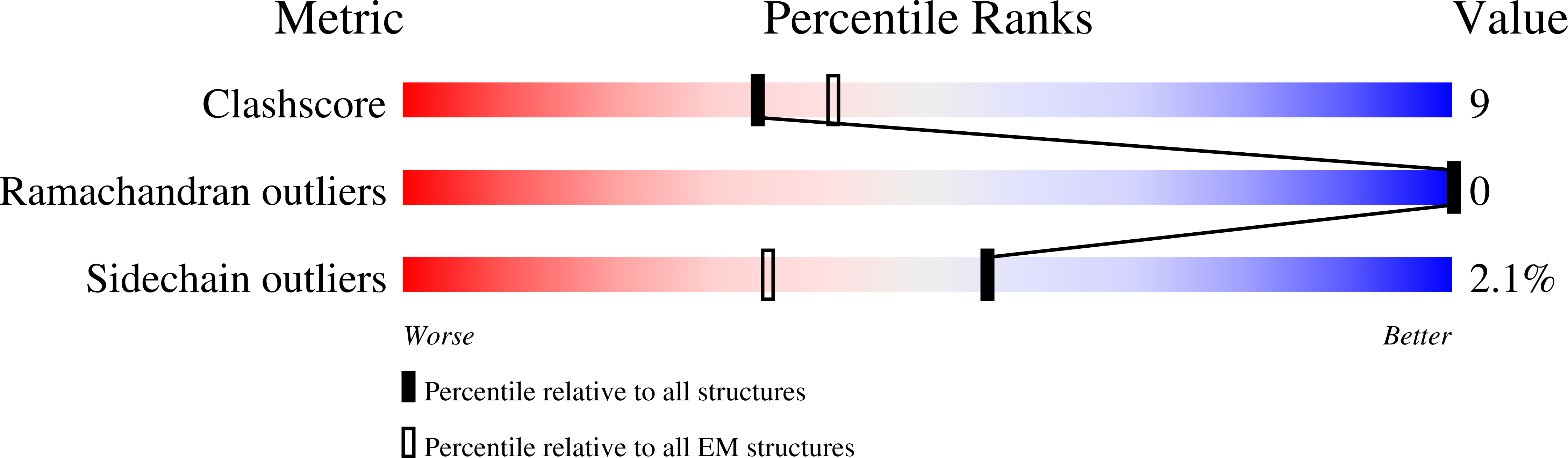
Deposition Date
2023-08-16
Release Date
2024-02-21
Last Version Date
2025-07-09
Entry Detail
PDB ID:
8Q7R
Keywords:
Title:
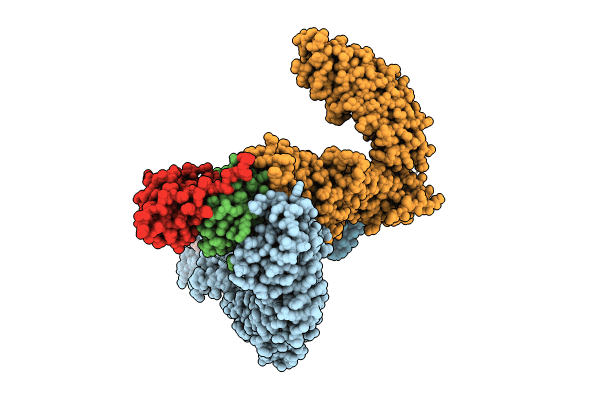
Ubiquitin ligation to substrate by a cullin-RING E3 ligase & Cdc34: NEDD8-CUL2-RBX1-ELOB/C-FEM1C with trapped UBE2R2~donor UB-Sil1 peptide
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.71 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE