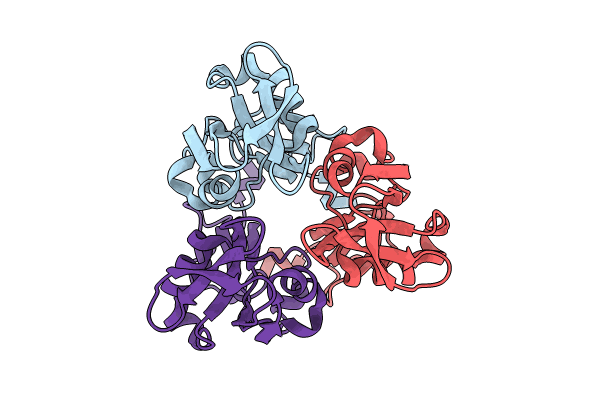
Deposition Date
2023-08-06
Release Date
2023-12-27
Last Version Date
2024-11-06
Entry Detail
PDB ID:
8Q4E
Keywords:
Title:
Structure of Legionella pneumophila Lcl C-terminal domain
Biological Source:
Source Organism:
Legionella pneumophila 130b (Taxon ID: 866628)
Host Organism:
Method Details:
Experimental Method:
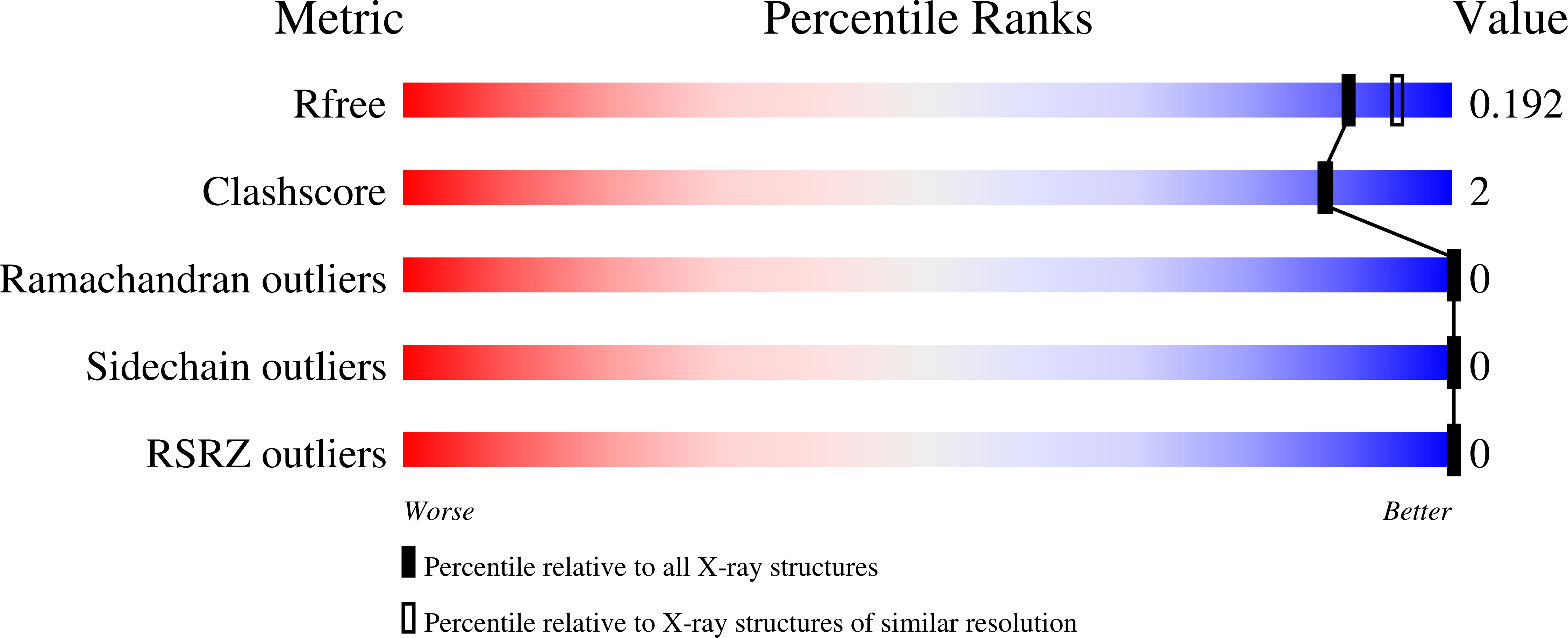
Resolution:
1.90 Å
R-Value Free:
0.18
R-Value Work:
0.15
Space Group:
C 1 2 1