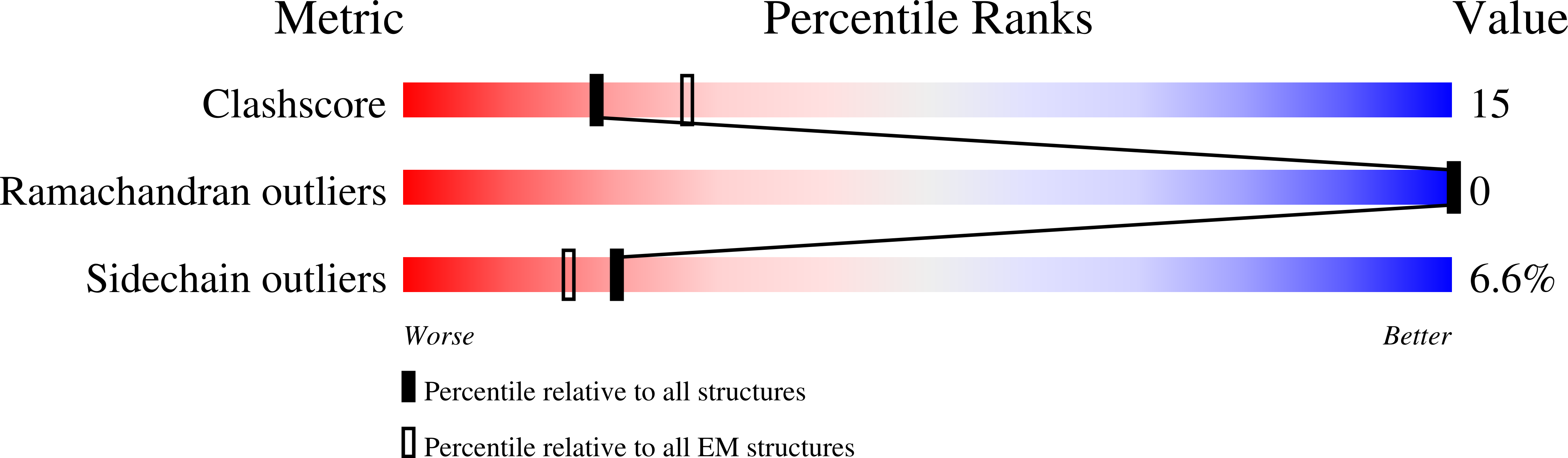
Deposition Date
2023-08-04
Release Date
2023-09-27
Last Version Date
2024-11-27
Entry Detail
PDB ID:
8Q3P
Keywords:
Title:
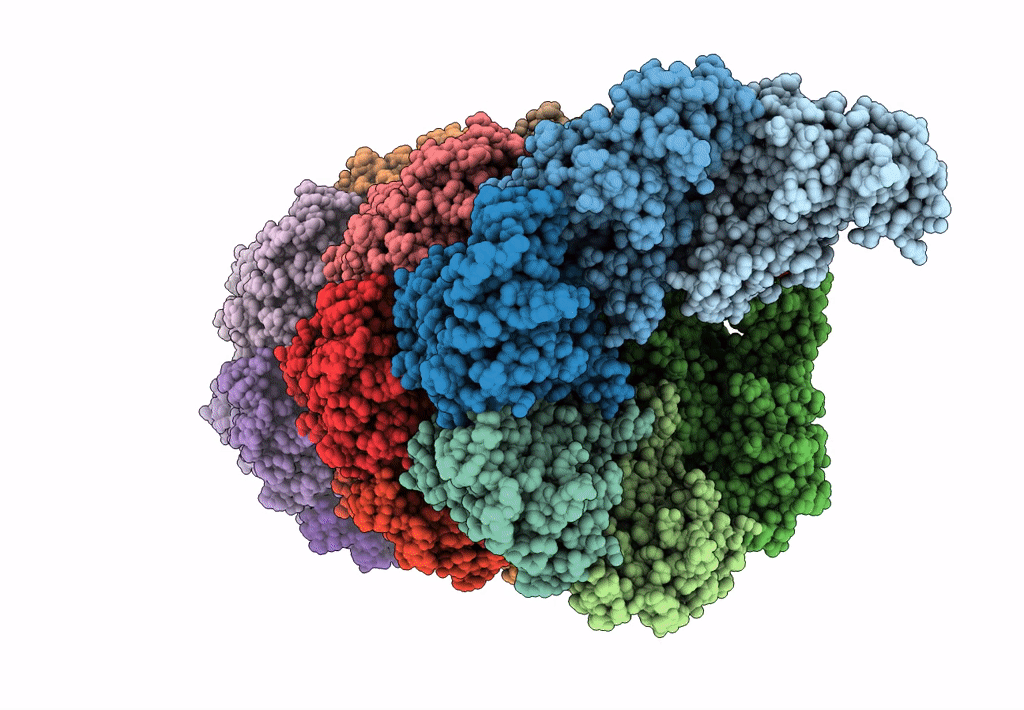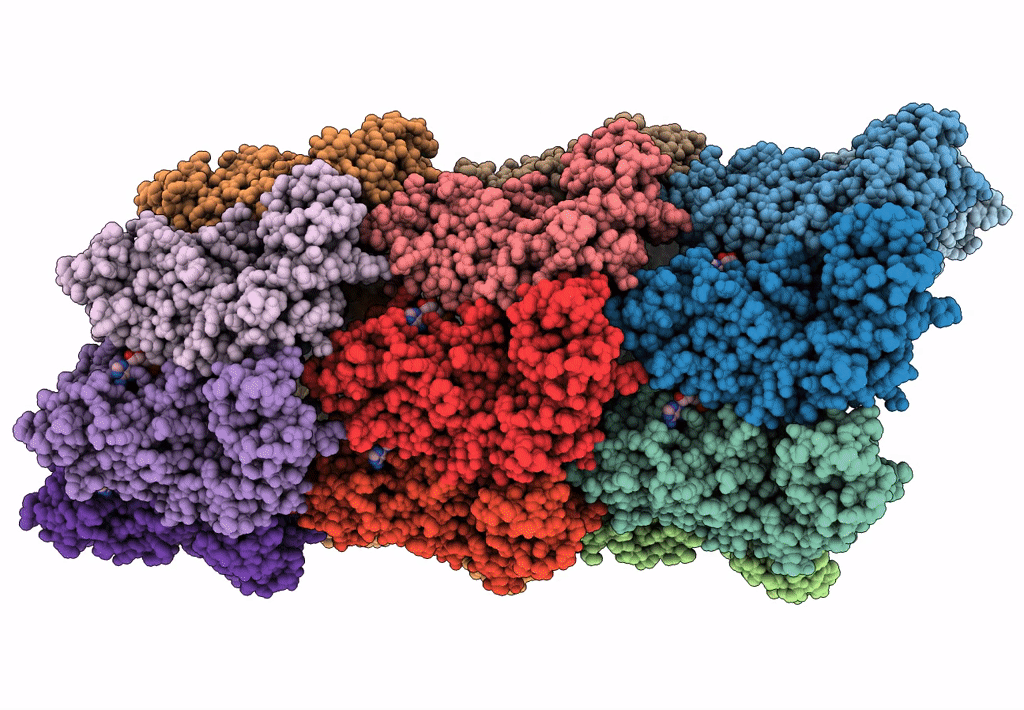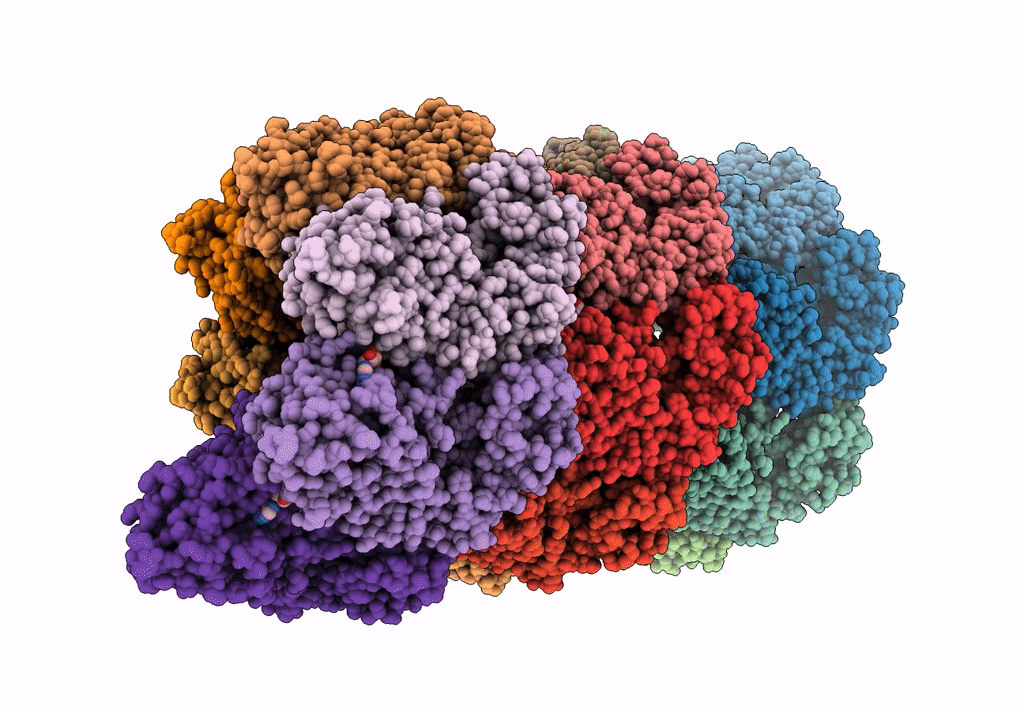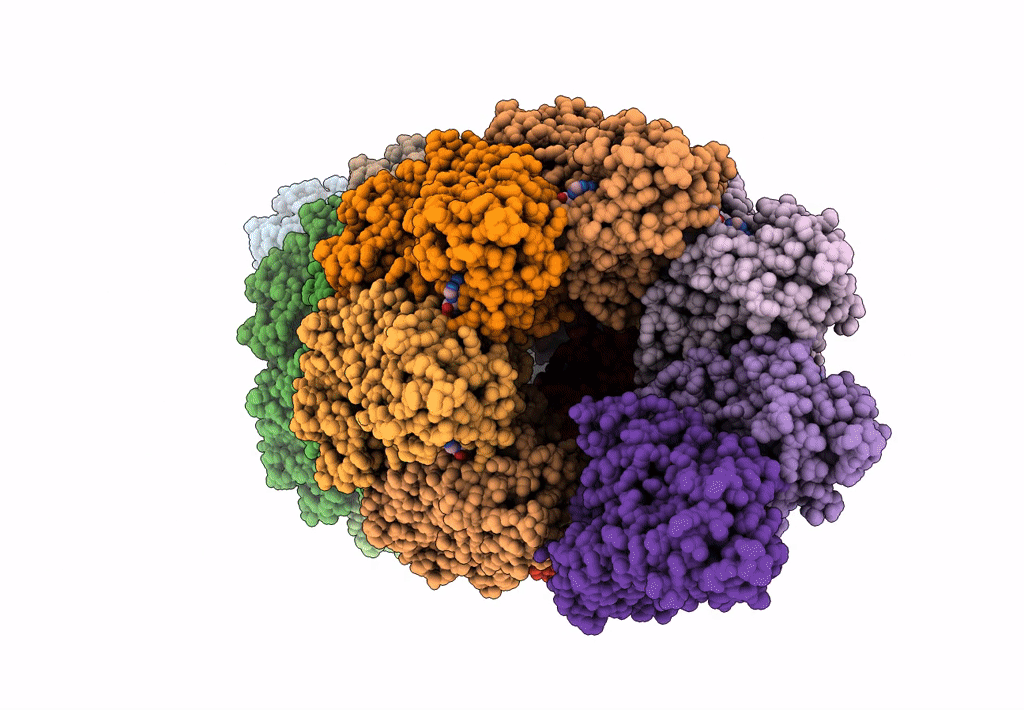
Bacterial transcription termination factor Rho G150D mutant bound to ADP; C-terminal 8xHis-tag
Biological Source:
Source Organism(s):
Escherichia (Taxon ID: 561)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
HELICAL