Deposition Date
2023-07-27
Release Date
2023-12-27
Last Version Date
2025-01-22
Entry Detail
PDB ID:
8PZP
Keywords:
Title:
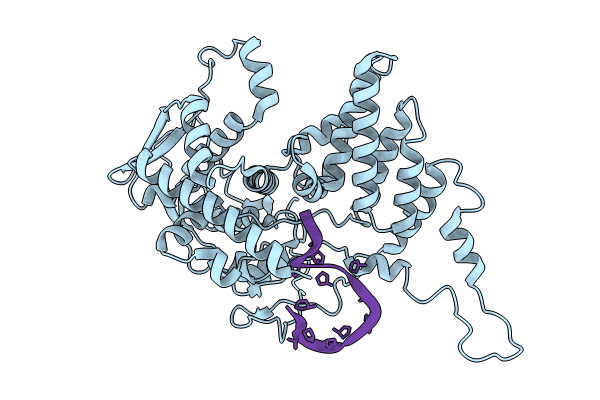
Model for influenza A virus helical ribonucleoprotein-like structure
Biological Source:
Source Organism(s):
Influenza A virus (A/WSN/1933(H1N1)) (Taxon ID: 382835)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
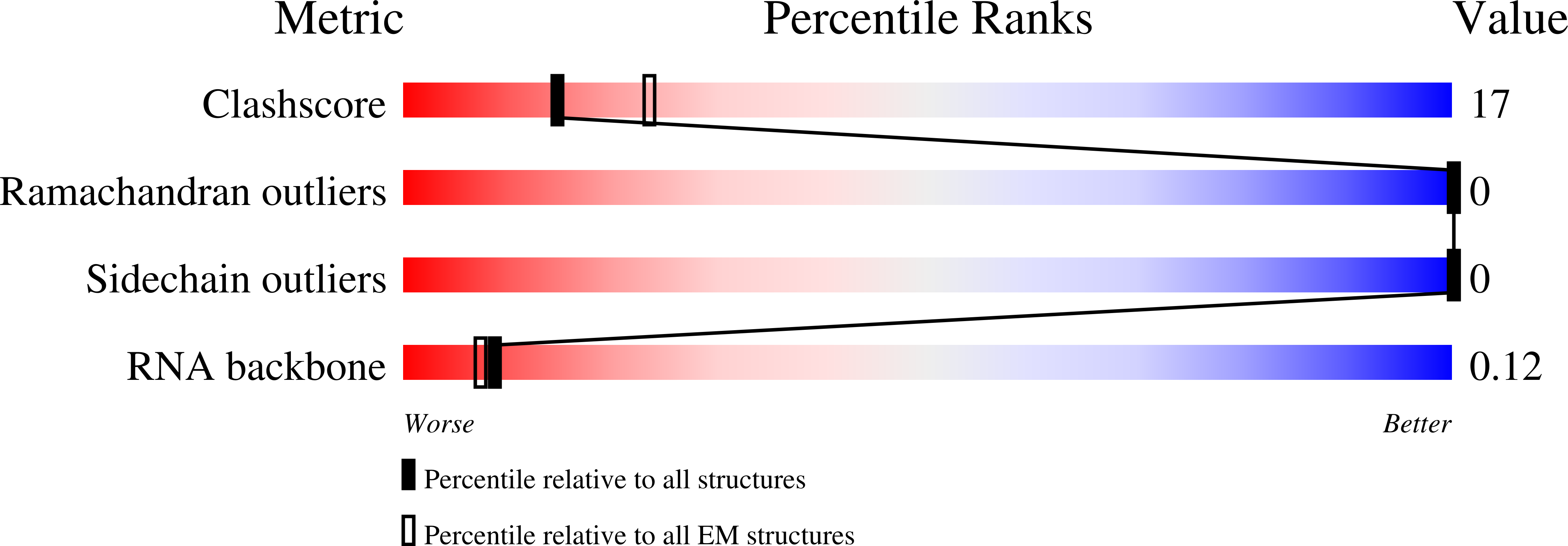
Resolution:
8.70 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL