Deposition Date
2023-07-17
Release Date
2025-03-05
Last Version Date
2025-07-16
Entry Detail
PDB ID:
8PUK
Keywords:
Title:
ChiLob 7/4 H2 HC-T219C/C224S Kappa LC-E123C/C214S F(ab')2
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
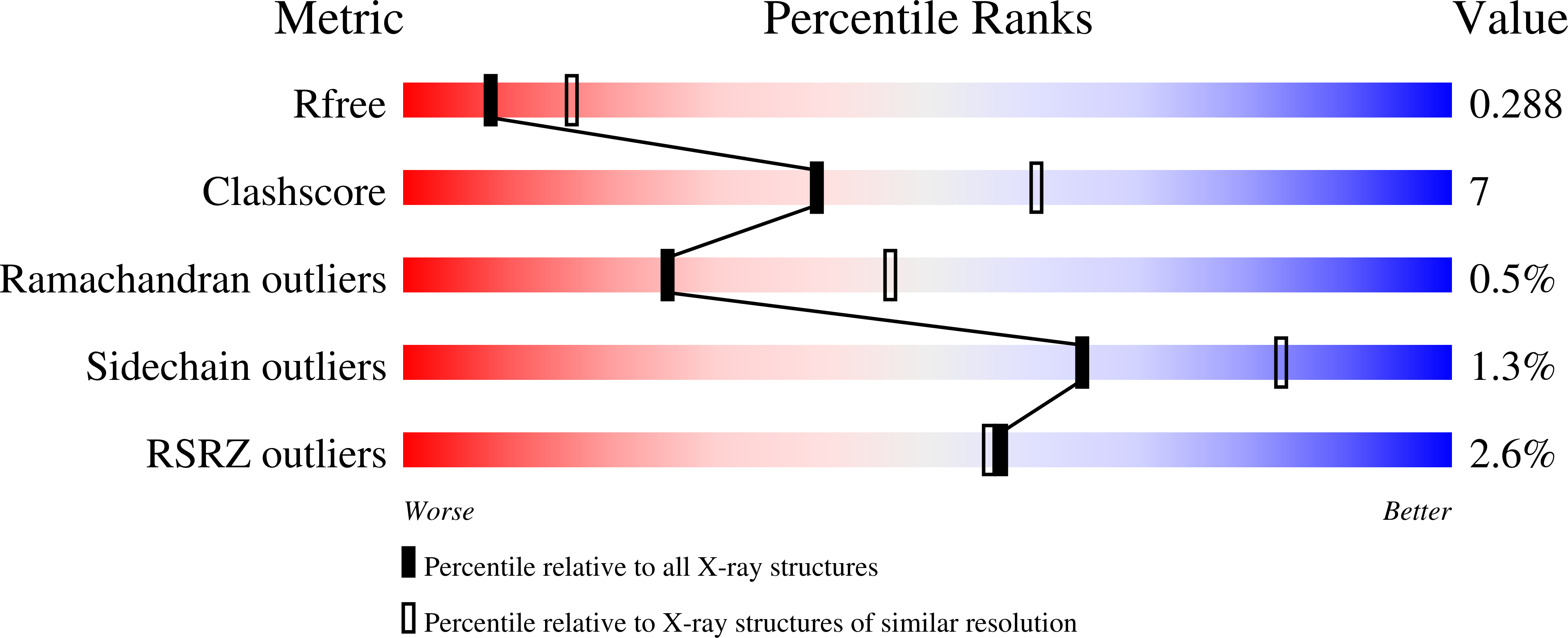
Resolution:
2.67 Å
R-Value Free:
0.28
R-Value Work:
0.24
Space Group:
P 3 2 1