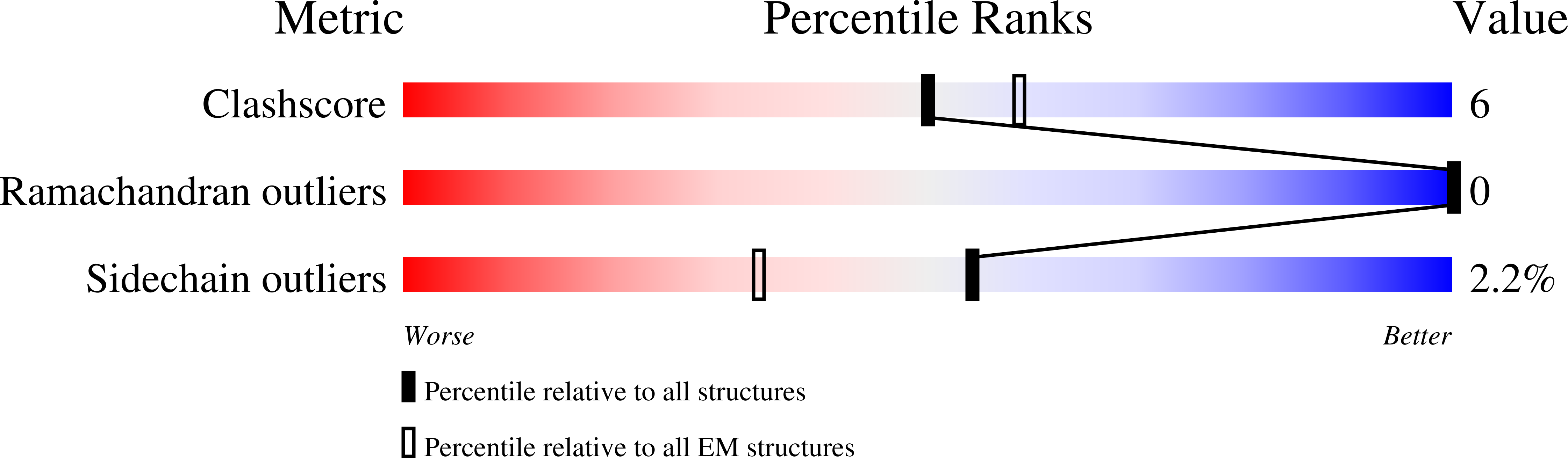
Deposition Date
2023-06-26
Release Date
2024-05-01
Last Version Date
2024-05-01
Entry Detail
PDB ID:
8PK9
Keywords:
Title:
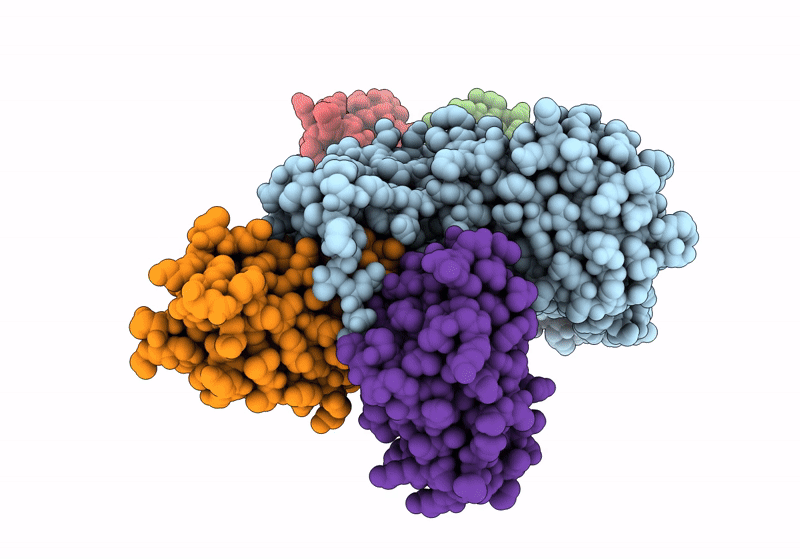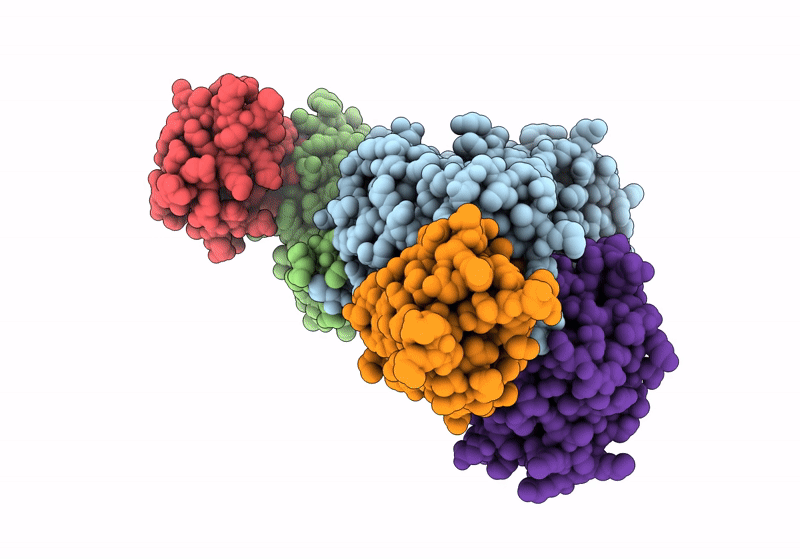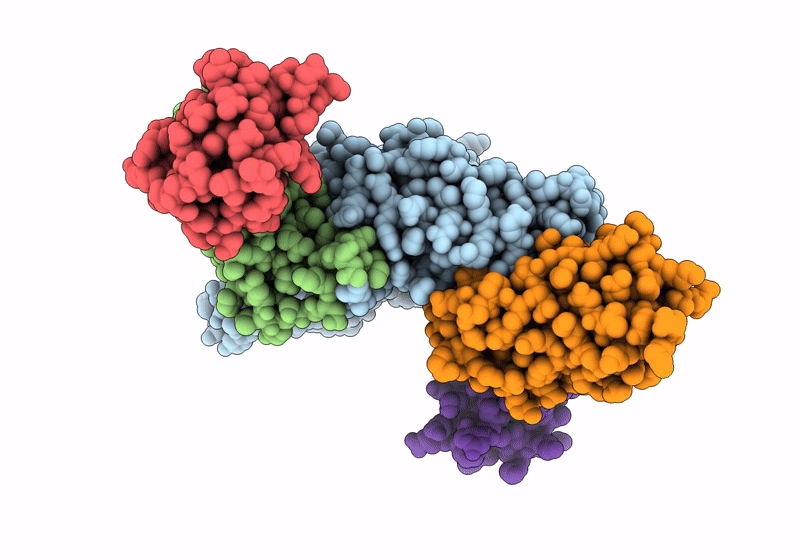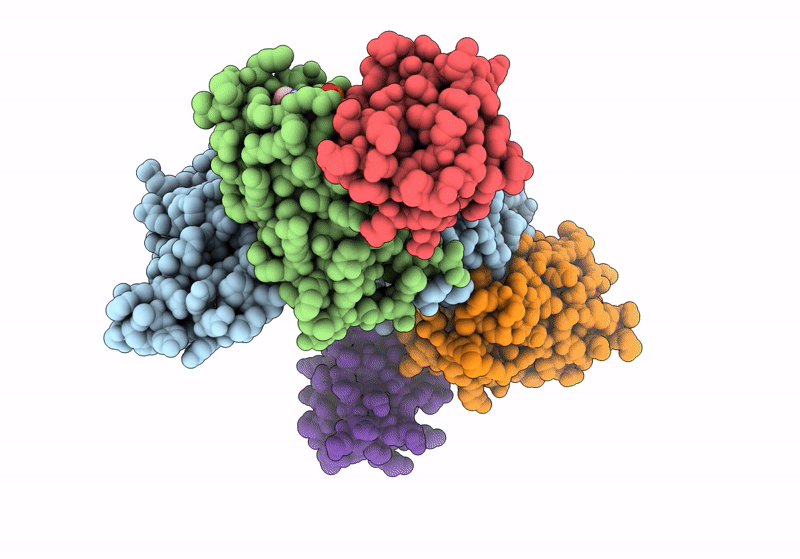
Structure of the human mitochondrial iron-sulfur cluster biosynthesis complex during persulfide transfer (persulfide on NFS1 and ISCU2)
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Escherichia coli BL21(DE3) (Taxon ID: 469008)
Escherichia coli BL21(DE3) (Taxon ID: 469008)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.58 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE