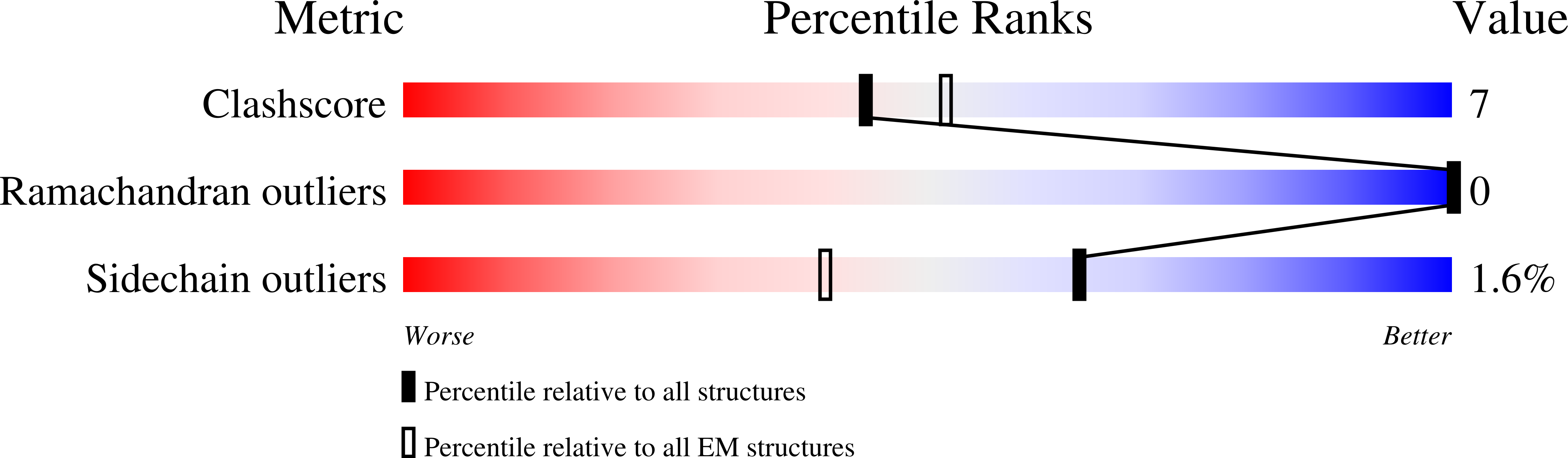
Deposition Date
2023-06-09
Release Date
2023-10-04
Last Version Date
2024-01-31
Entry Detail
PDB ID:
8PBB
Keywords:
Title:
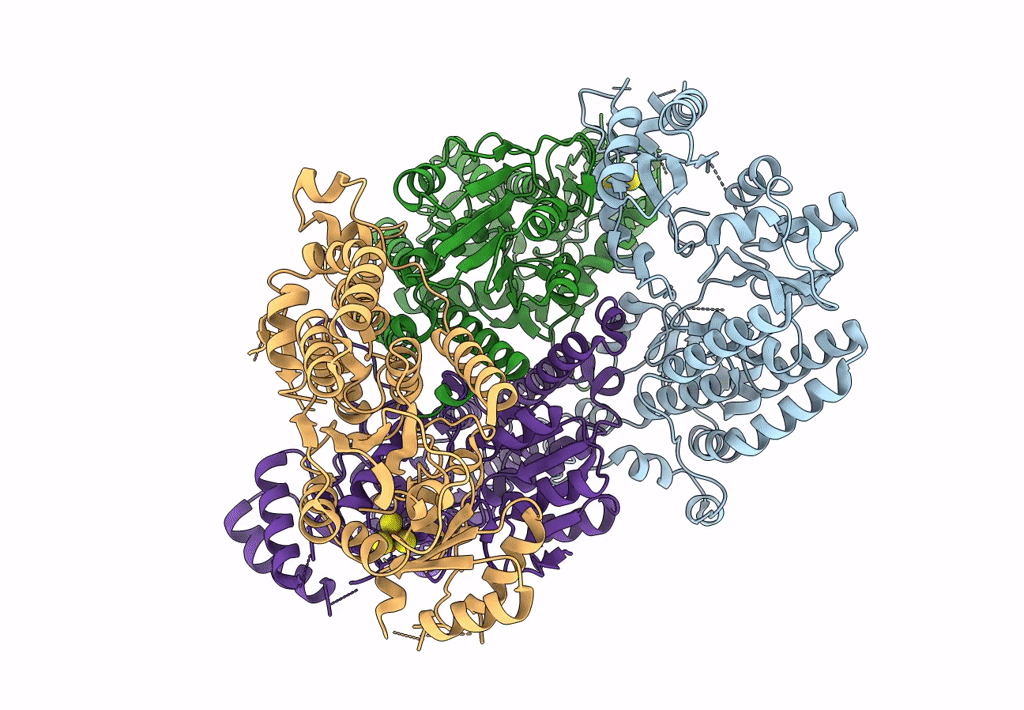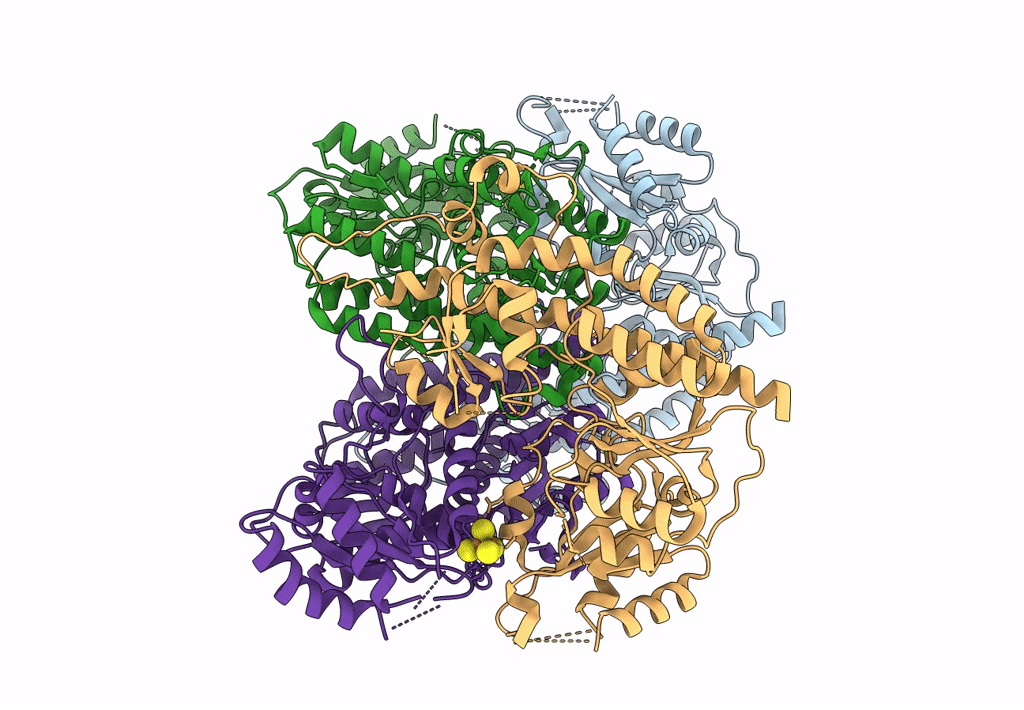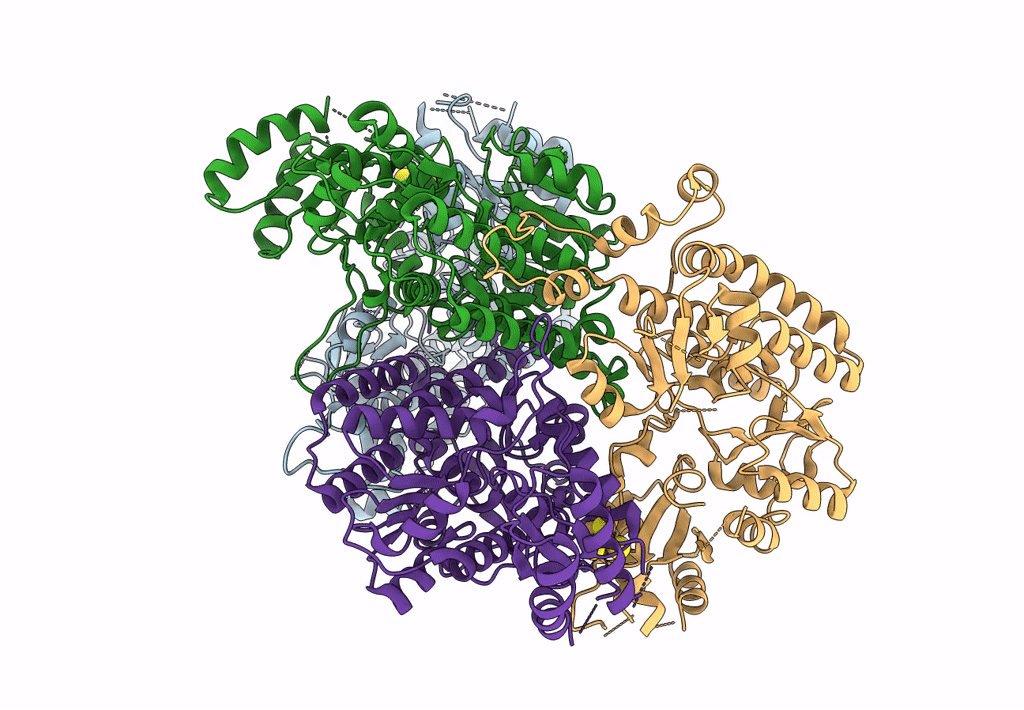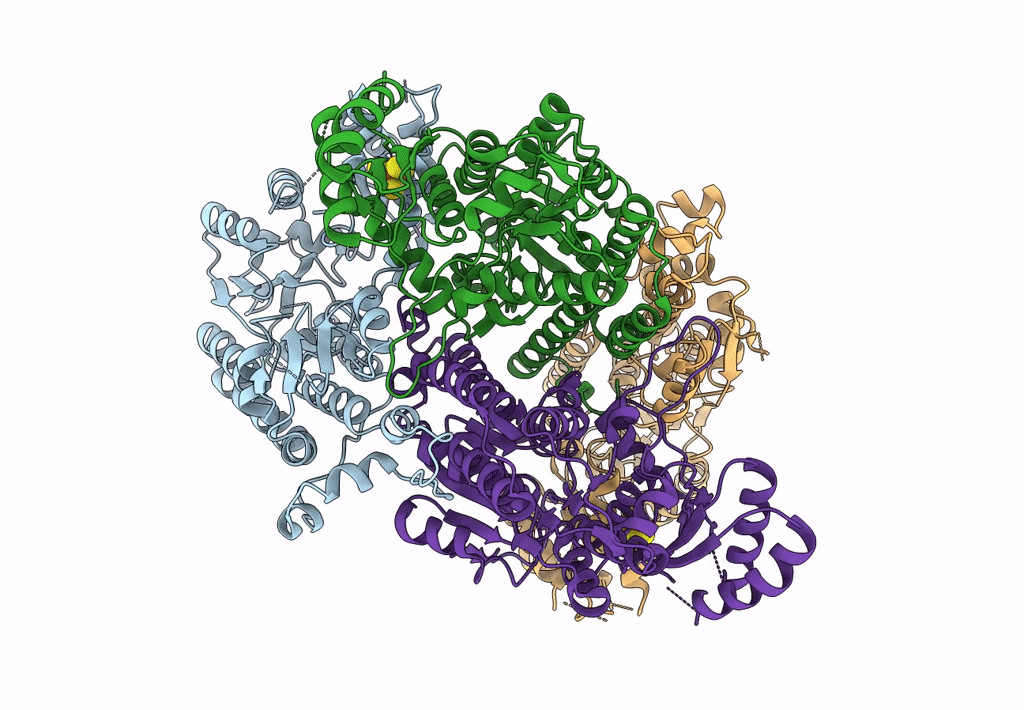
CHAPSO treated partial catalytic component (comprising only AnfD & AnfK, lacking AnfG and FeFeco) of iron nitrogenase from Rhodobacter capsulatus
Biological Source:
Source Organism(s):
Rhodobacter capsulatus SB 1003 (Taxon ID: 272942)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.49 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE