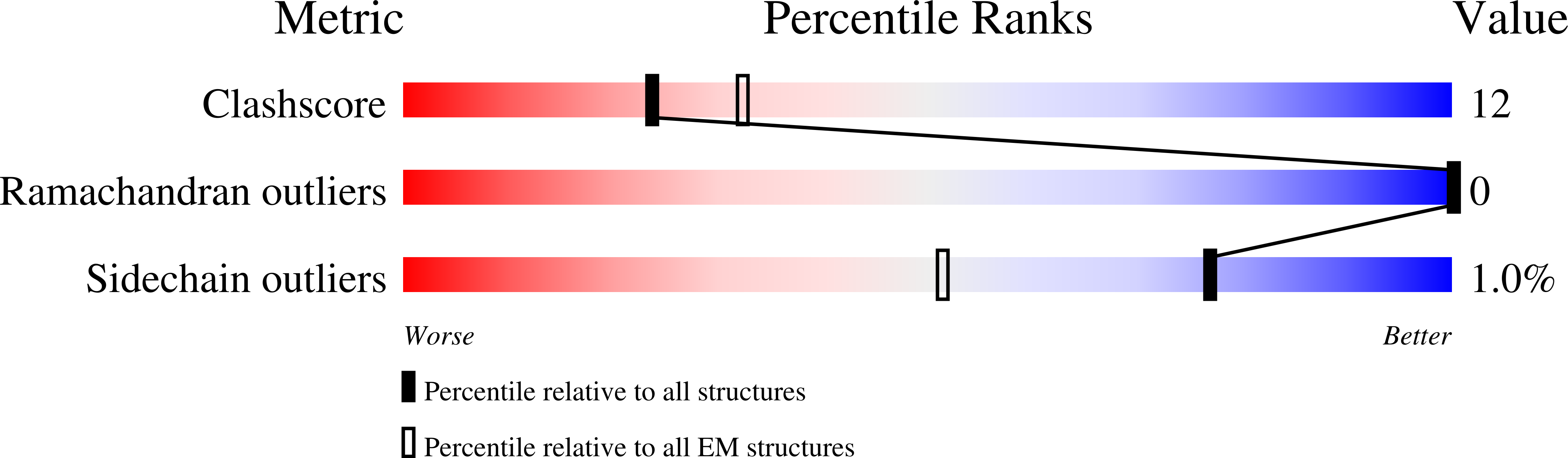
Deposition Date
2023-04-06
Release Date
2023-08-09
Last Version Date
2025-07-09
Entry Detail
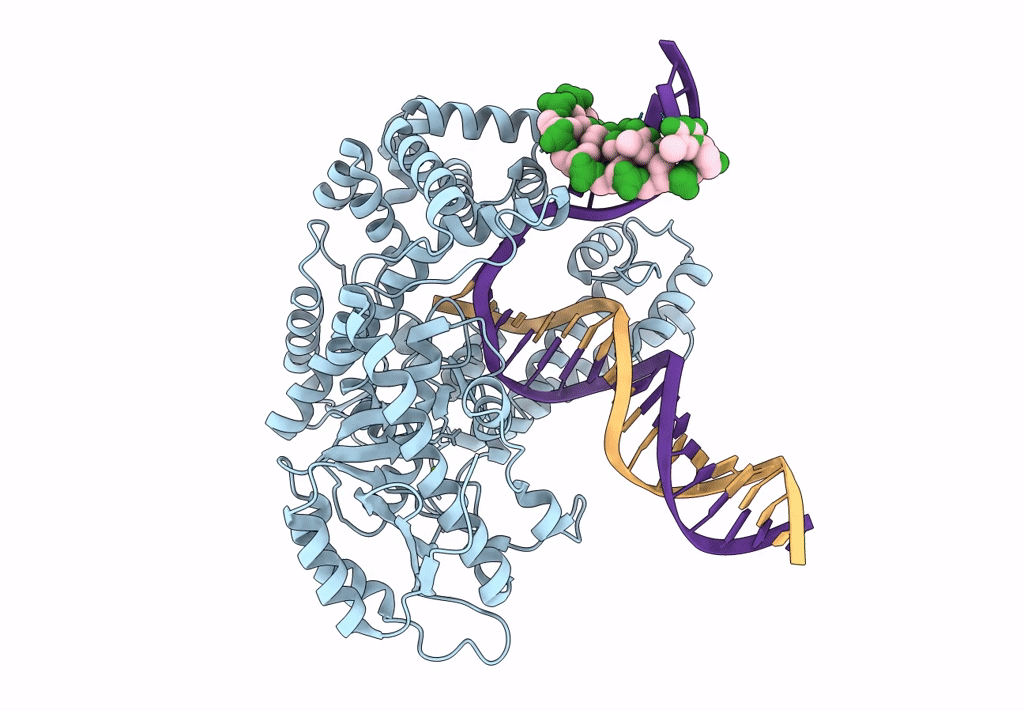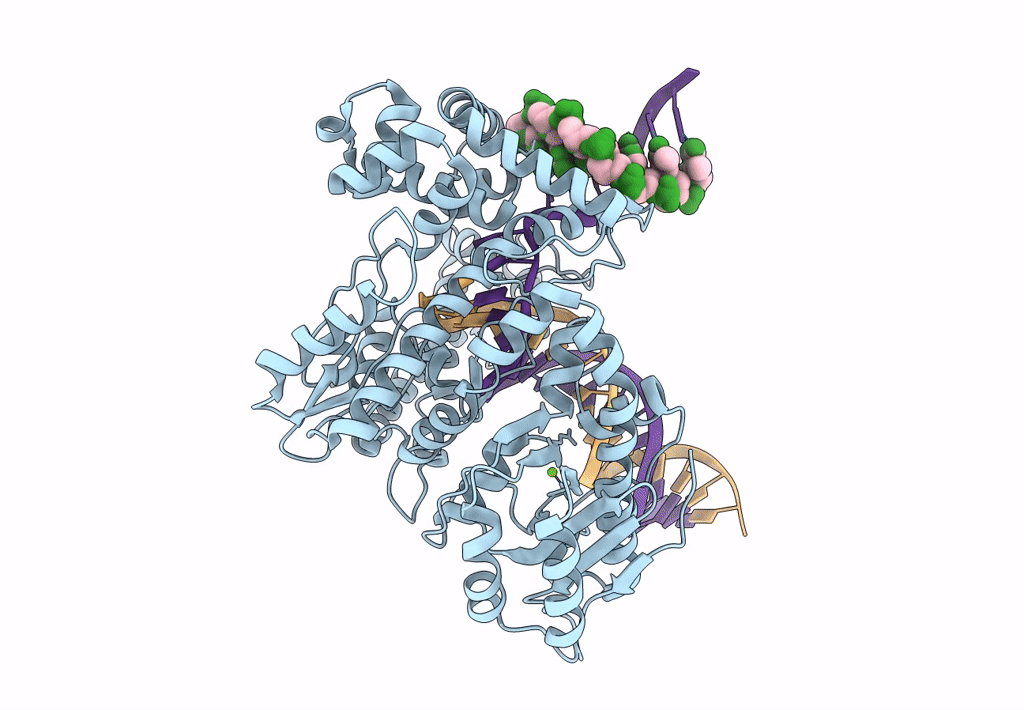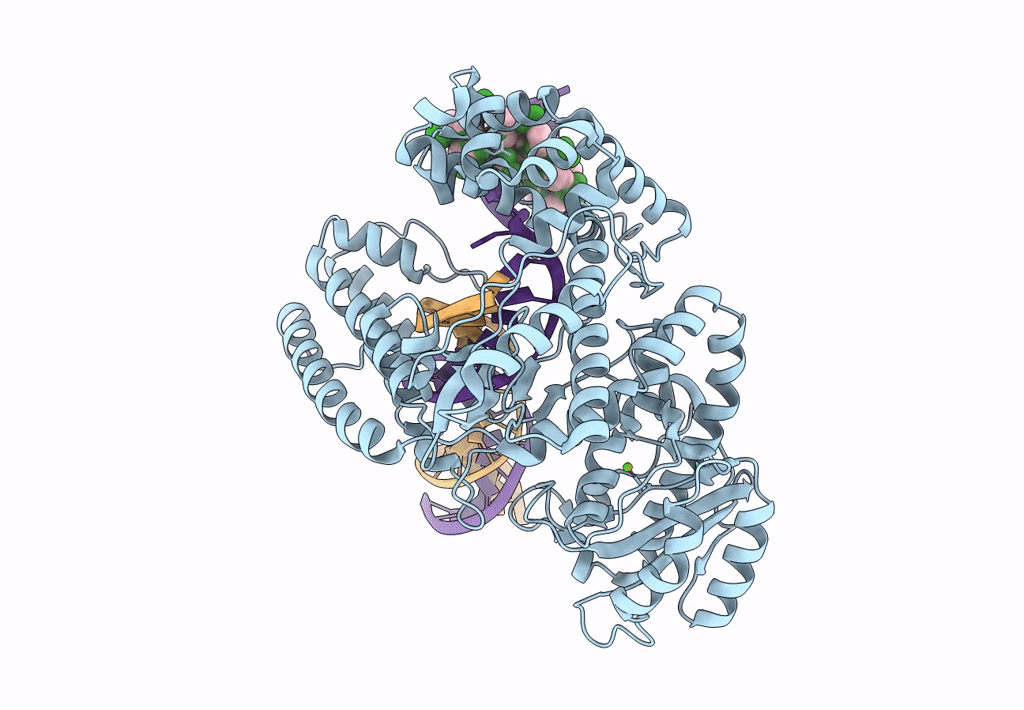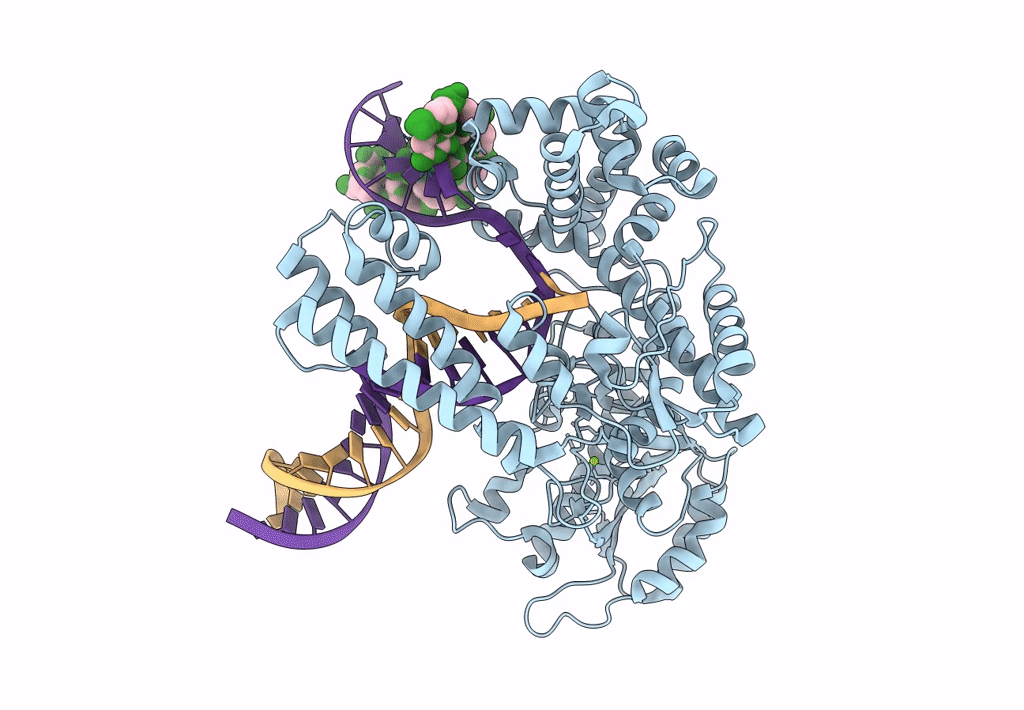
PDB ID:
8OOY
Keywords:
Title:
Pol I bound to extended and displaced DNA section - open conformation
Biological Source:
Source Organism(s):
Escherichia coli 'BL21-Gold(DE3)pLysS AG' (Taxon ID: 866768)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE