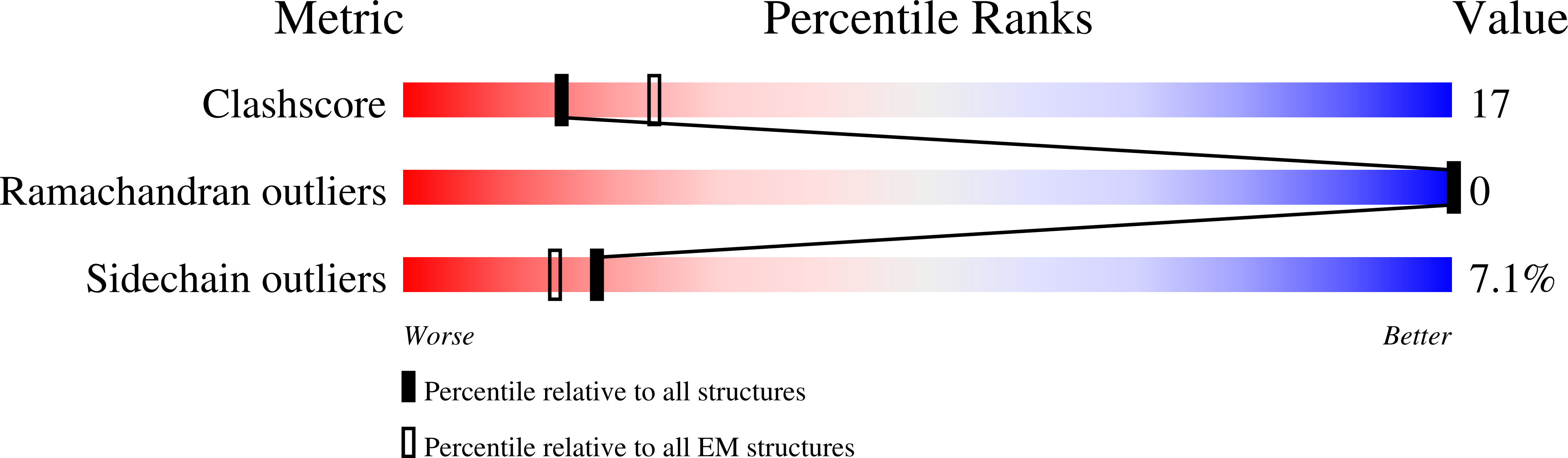
Deposition Date
2023-03-23
Release Date
2023-05-31
Last Version Date
2024-07-24
Entry Detail
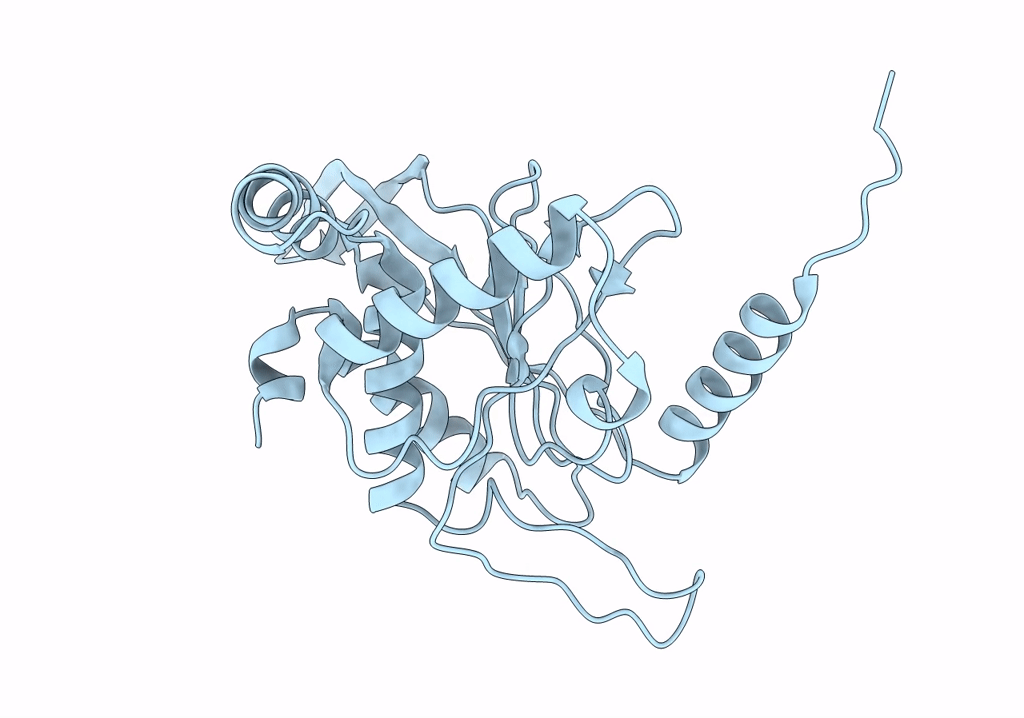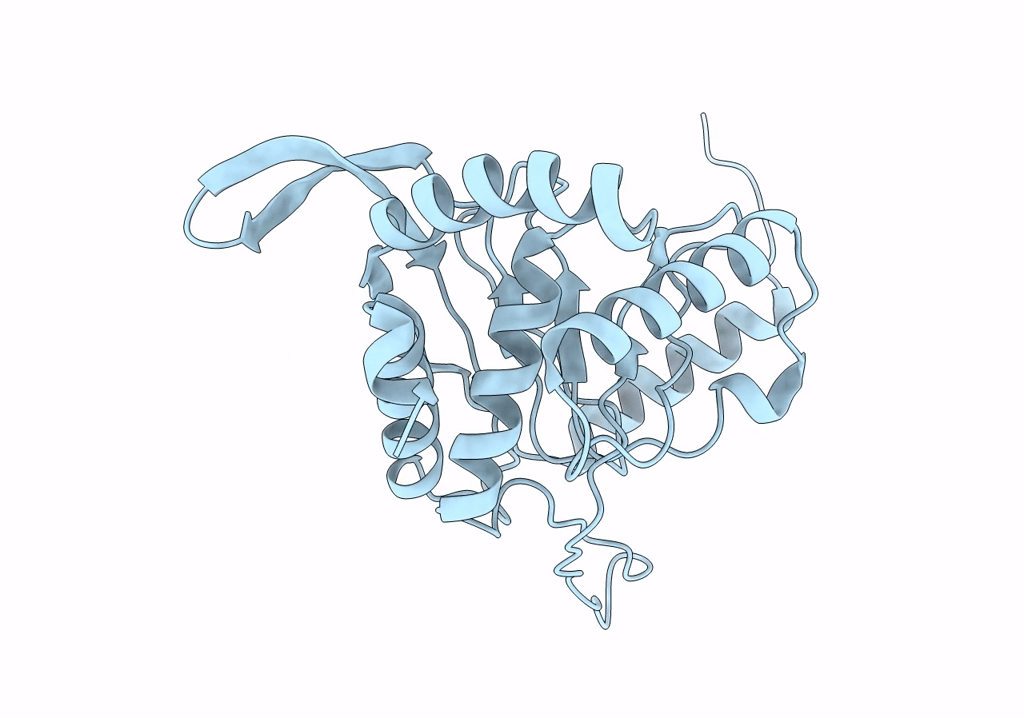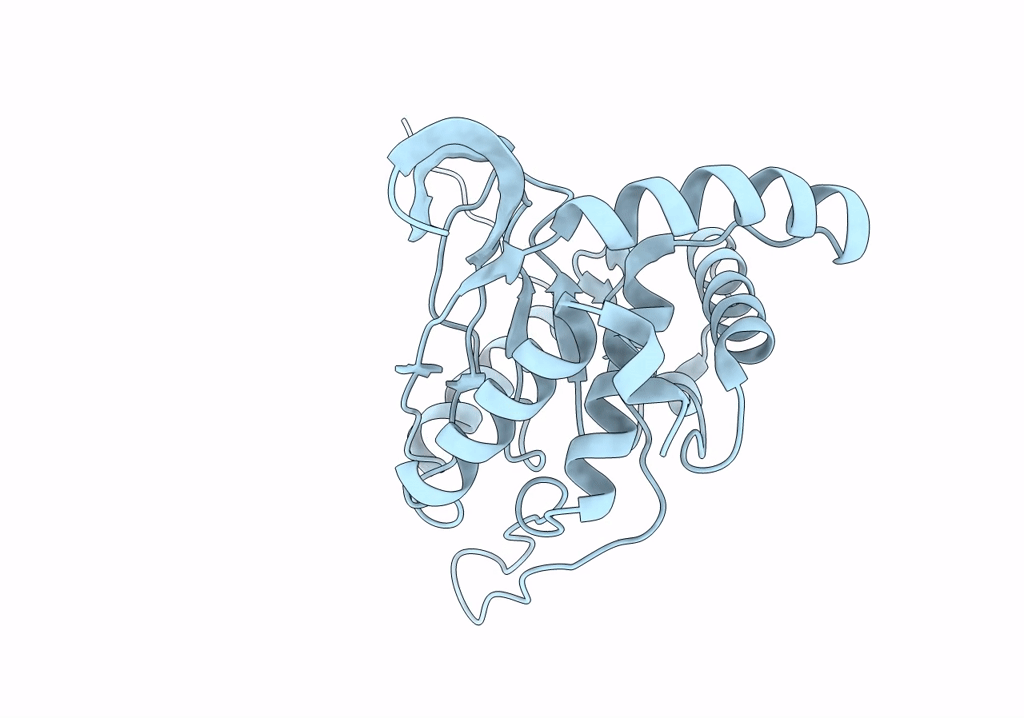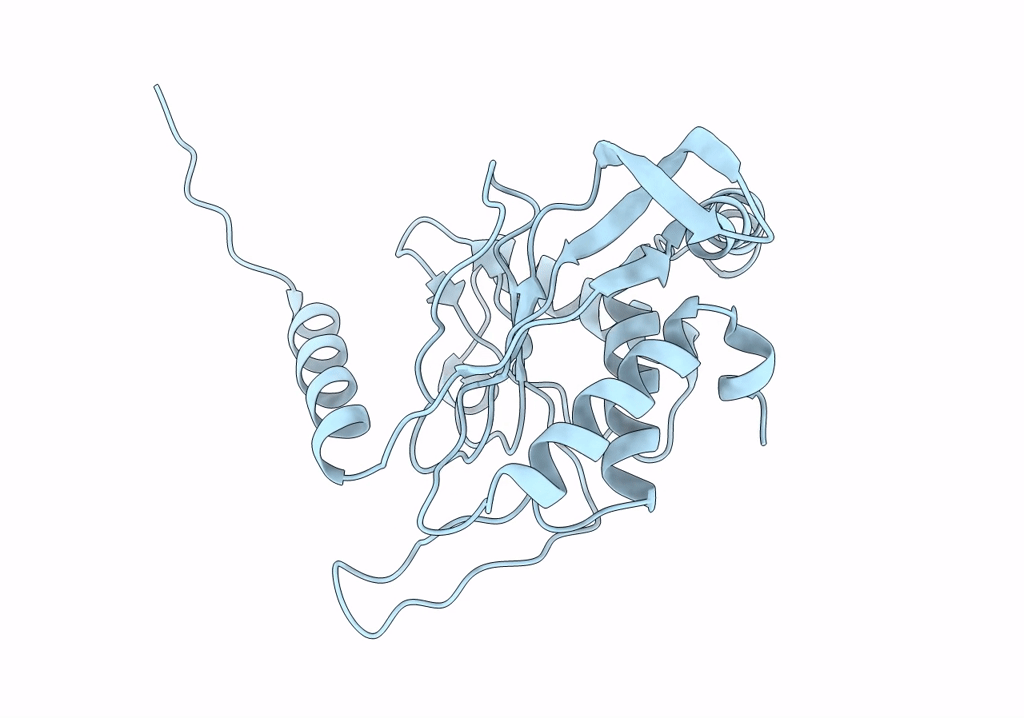
PDB ID:
8OIU
Keywords:
Title:
Cryo-EM reconstruction of the native 24-mer E2o core of the 2-oxoglutarate dehydrogenase complex of C. thermophilum at 3.35 A resolution
Biological Source:
Source Organism(s):
Thermochaetoides thermophila DSM 1495 (Taxon ID: 759272)
Method Details:
Experimental Method:
Resolution:
3.35 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE