Deposition Date
2023-03-21
Release Date
2023-04-26
Last Version Date
2024-07-24
Entry Detail
PDB ID:
8OHS
Keywords:
Title:
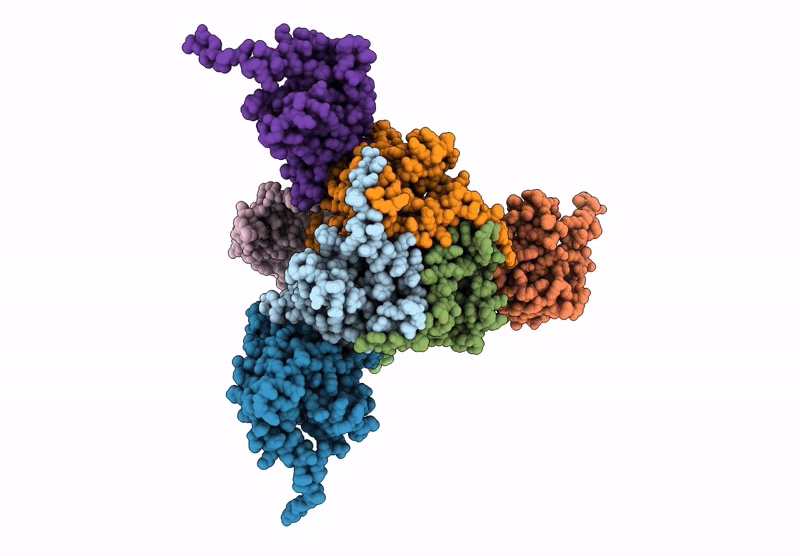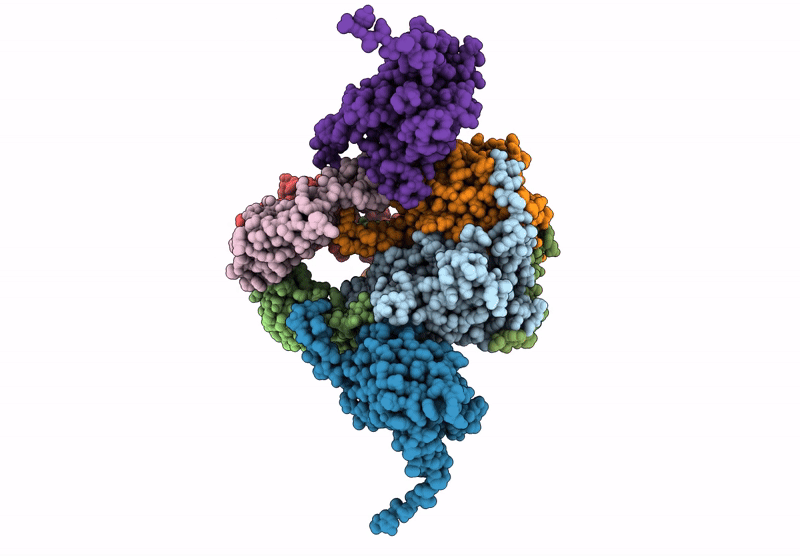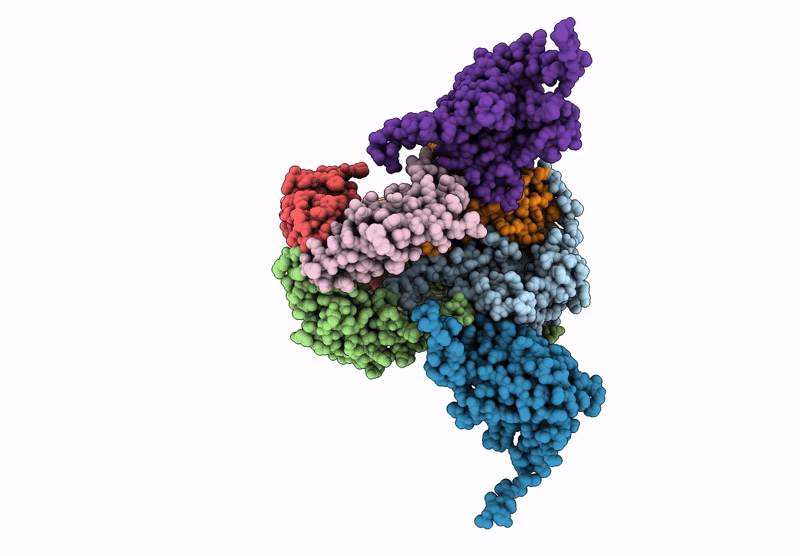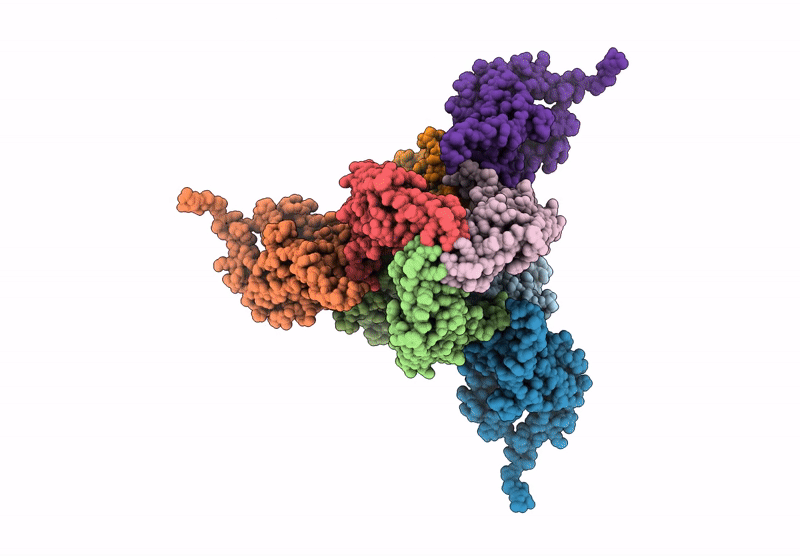
Core-binding domain of fungal E3-binding domain bound to the native pyruvate dehydrogenase E2 core
Biological Source:
Source Organism(s):
Neurospora crassa (Taxon ID: 5141)
Method Details:
Experimental Method:
Resolution:
4.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


