Deposition Date
2023-03-14
Release Date
2024-01-31
Last Version Date
2024-11-20
Entry Detail
PDB ID:
8IPC
Keywords:
Title:
The recombinant NZ-1 Fab complexed with the PDZ tandem fragment of A. aeolicus S2P homolog with the PA14 tag inserted between the residues 181 and 184
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
Aquifex aeolicus VF5 (Taxon ID: 224324)
Aquifex aeolicus VF5 (Taxon ID: 224324)
Expression System(s):
Method Details:
Experimental Method:
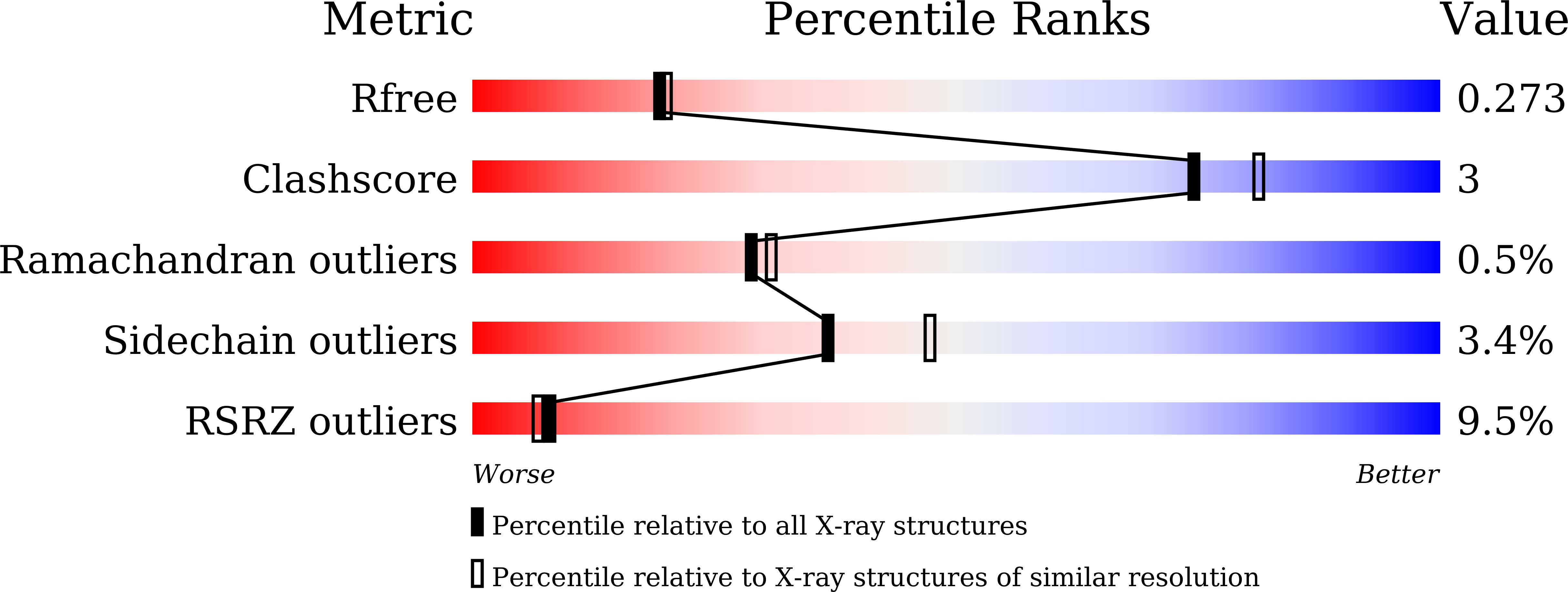
Resolution:
2.20 Å
R-Value Free:
0.26
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 21 21 21