Deposition Date
2023-03-03
Release Date
2024-01-10
Last Version Date
2024-11-13
Entry Detail
PDB ID:
8ILE
Keywords:
Title:
The crystal structure of dGTPalphaSe-Rp:DNApre-II:Pol X substrate ternary complex
Biological Source:
Source Organism(s):
African swine fever virus (strain Badajoz 1971 Vero-adapted) (Taxon ID: 10498)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
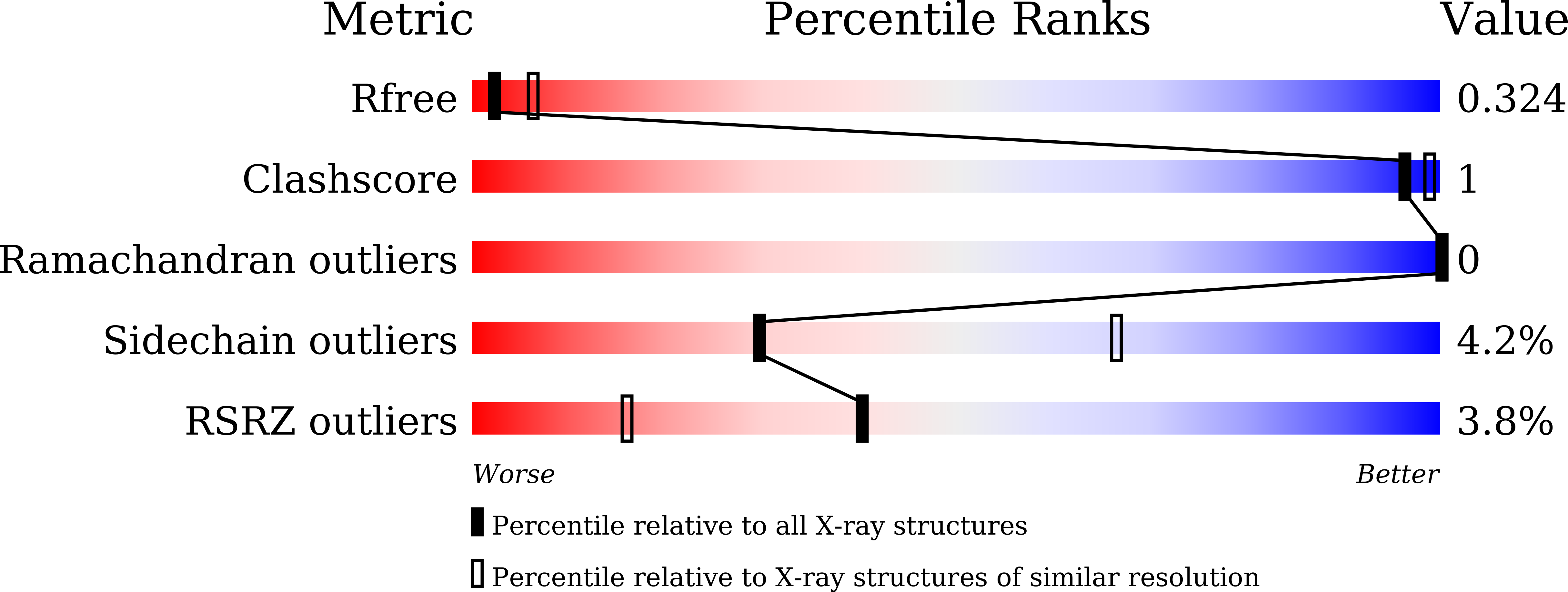
Resolution:
3.00 Å
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 21 21 21