Deposition Date
2023-02-21
Release Date
2023-07-12
Last Version Date
2025-03-12
Entry Detail
PDB ID:
8IGV
Keywords:
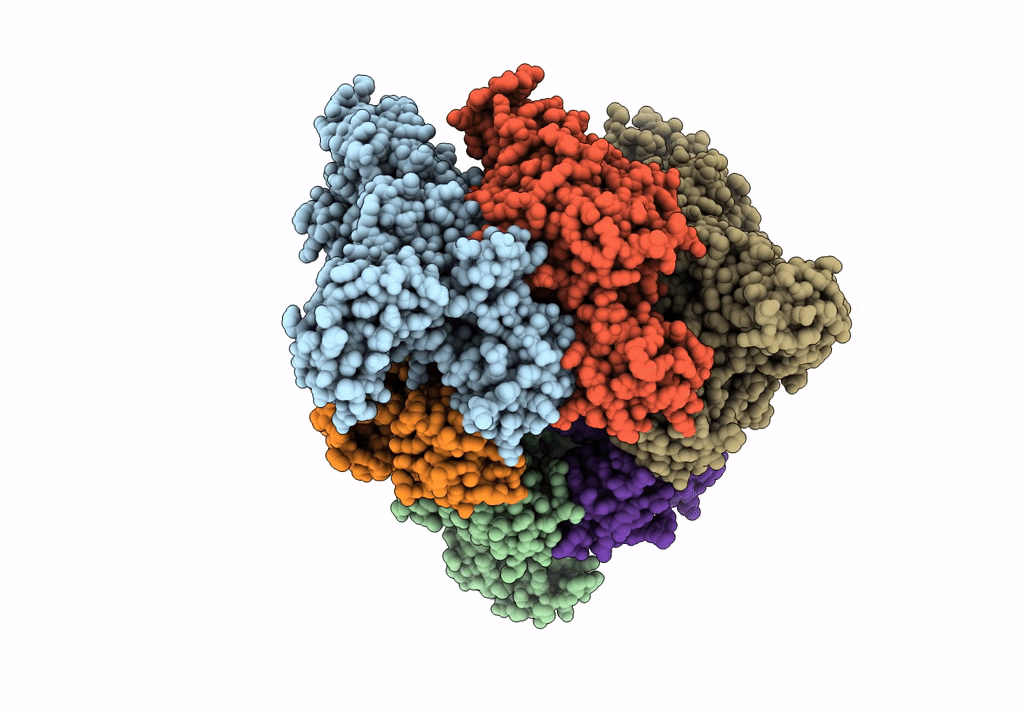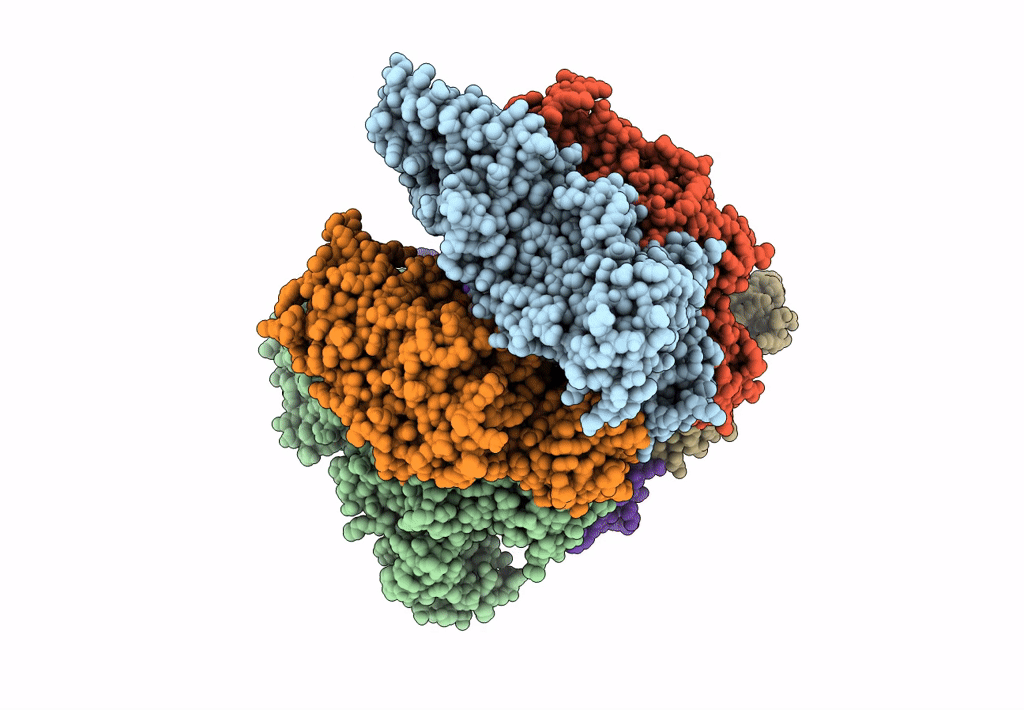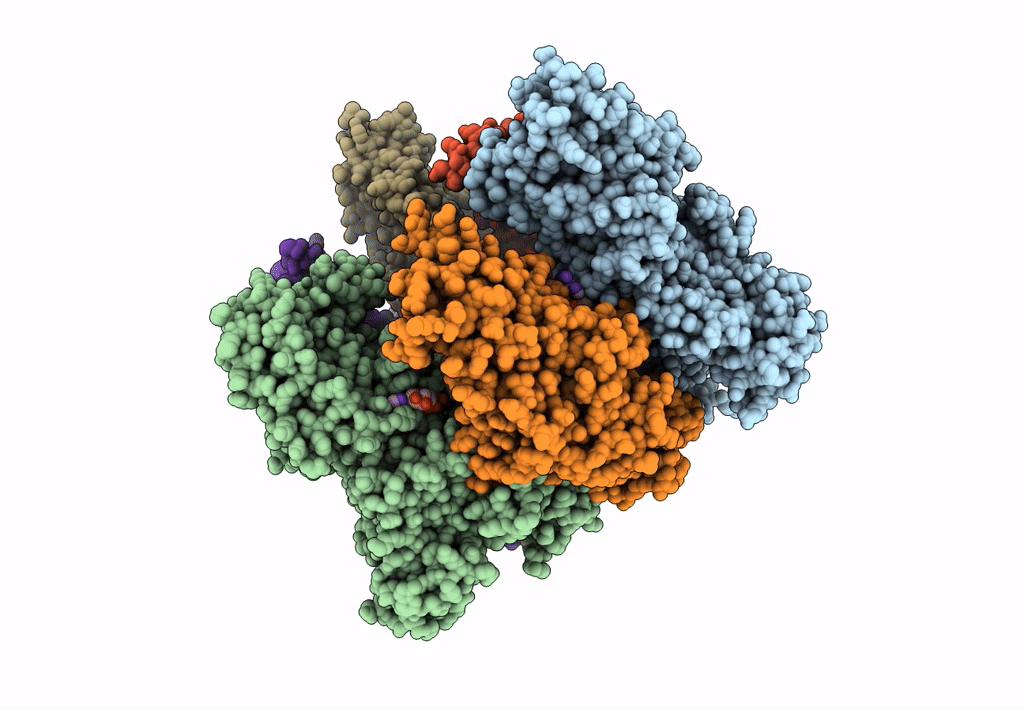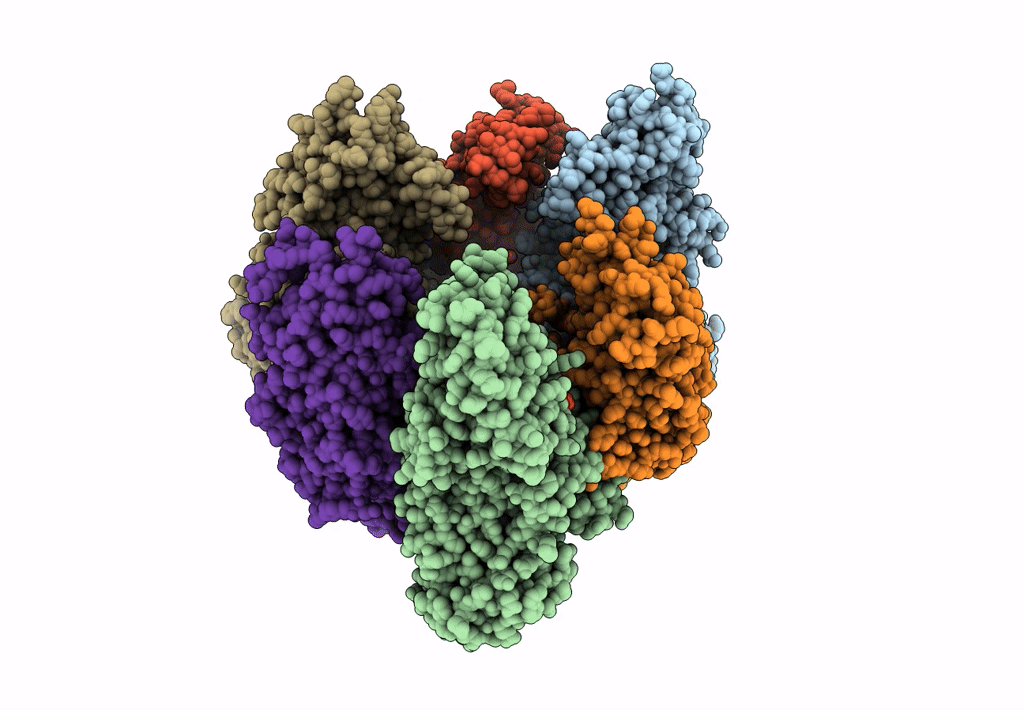
Title:
Hexameric Ring Complex of Engineered V1-ATPase bound to 5 ADPs: A3(De)3_(ADP-Pi)1cat(ADP)2cat,2non-cat
Biological Source:
Source Organism(s):
Enterococcus hirae ATCC 9790 (Taxon ID: 768486)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.15 Å
R-Value Free:
0.26
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 1 21 1


