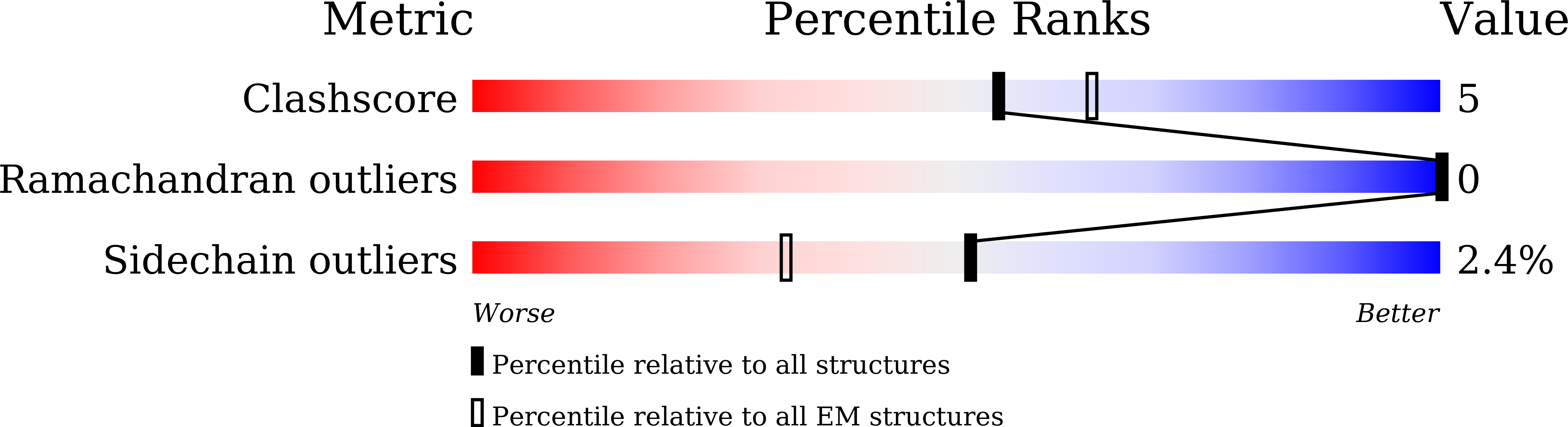Deposition Date
2023-02-21
Release Date
2023-05-17
Last Version Date
2023-08-16
Entry Detail
PDB ID:
8IGR
Keywords:
Title:
Cryo-EM structure of CII-dependent transcription activation complex
Biological Source:
Source Organism(s):
Escherichia coli (strain K12) (Taxon ID: 83333)
Escherichia phage Lambda (Taxon ID: 107710)
Escherichia phage Lambda (Taxon ID: 107710)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE