Deposition Date
2023-02-18
Release Date
2024-01-17
Last Version Date
2024-04-10
Entry Detail
PDB ID:
8IFK
Keywords:
Title:
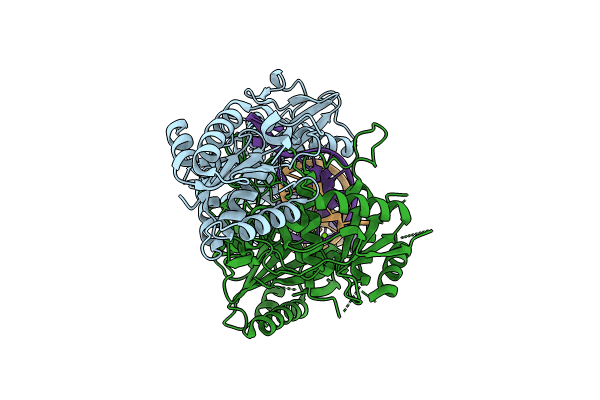
Cryo-EM structure of monomeric SPARTA gRNA-ssDNA target complex
Biological Source:
Source Organism(s):
Thermoflavifilum thermophilum (Taxon ID: 1393122)
Escherichia coli 'BL21-Gold(DE3)pLysS AG' (Taxon ID: 866768)
Escherichia coli 'BL21-Gold(DE3)pLysS AG' (Taxon ID: 866768)
Expression System(s):
Method Details:
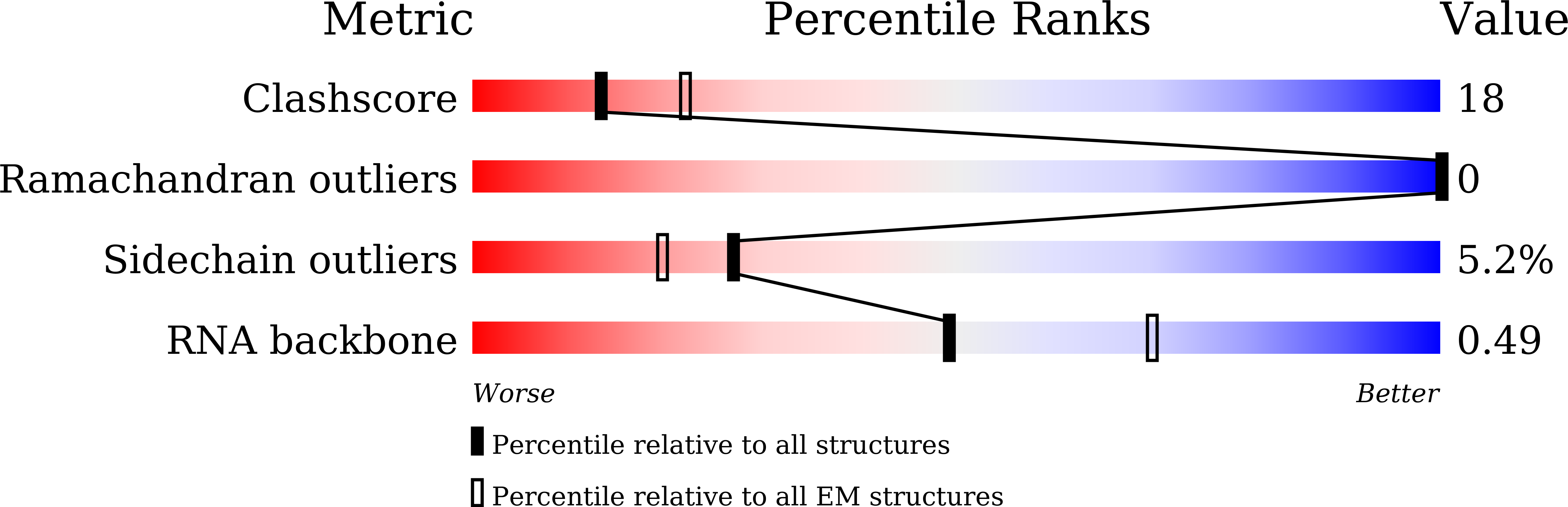
Experimental Method:
Resolution:
2.54 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE