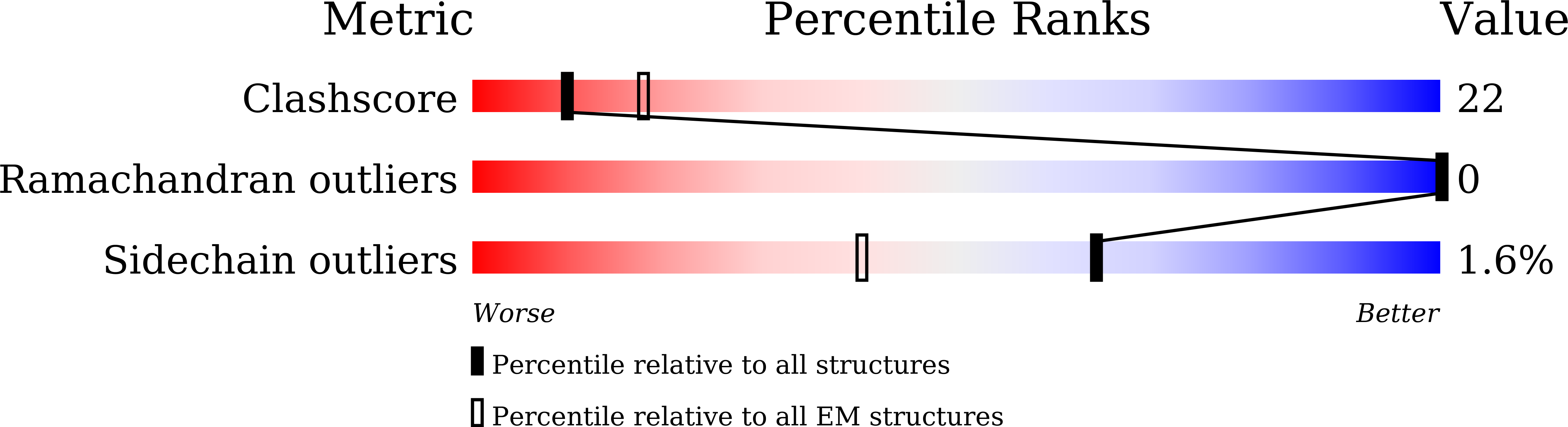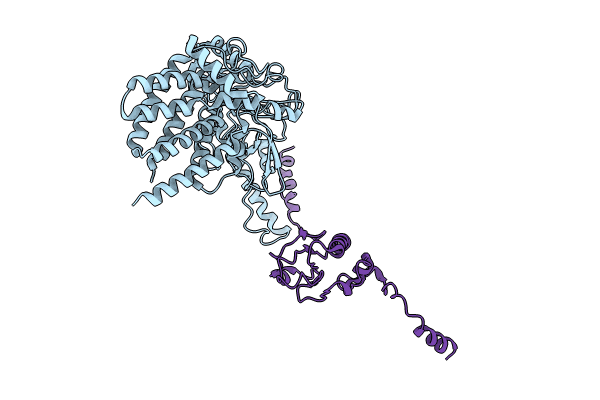
Deposition Date
2023-02-07
Release Date
2023-12-20
Last Version Date
2024-05-08
Entry Detail
PDB ID:
8I9Q
Keywords:
Title:
The focused refinement of CCT4-PhLP2A from TRiC-PhLP2A complex in the open state
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Host Organism:
Method Details:
Experimental Method:
Resolution:
4.22 Å
Aggregation State:
2D ARRAY
Reconstruction Method:
SINGLE PARTICLE