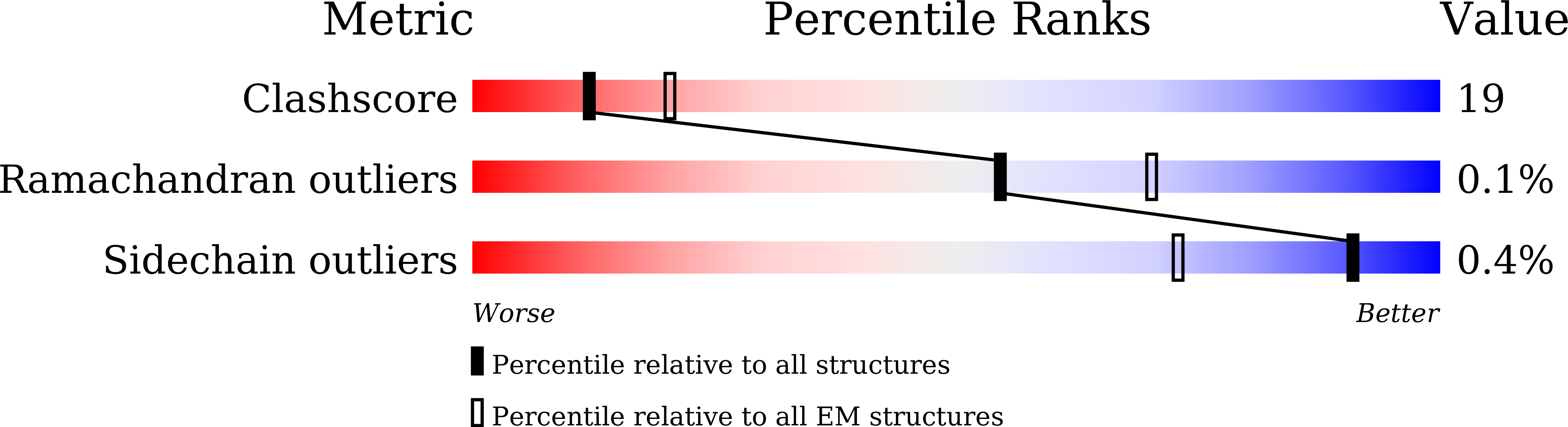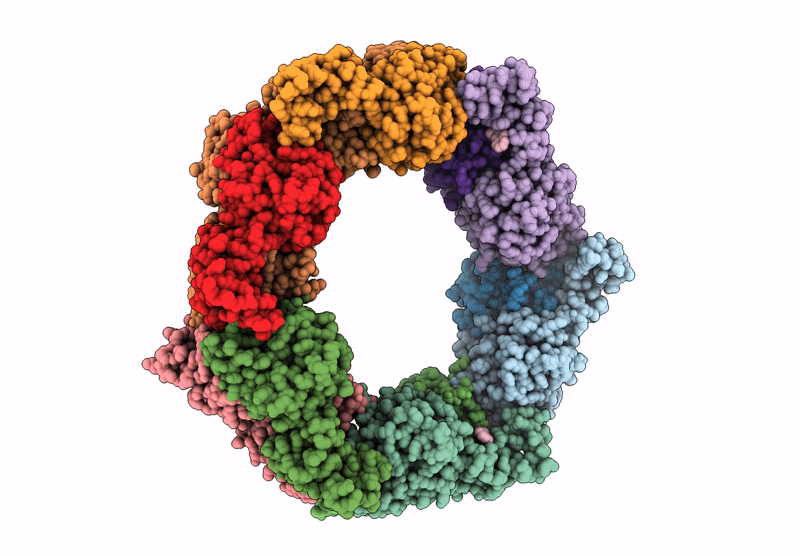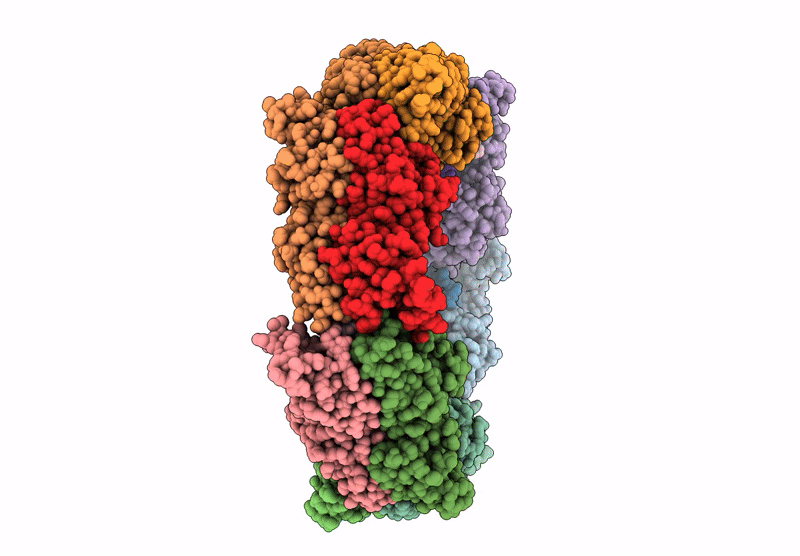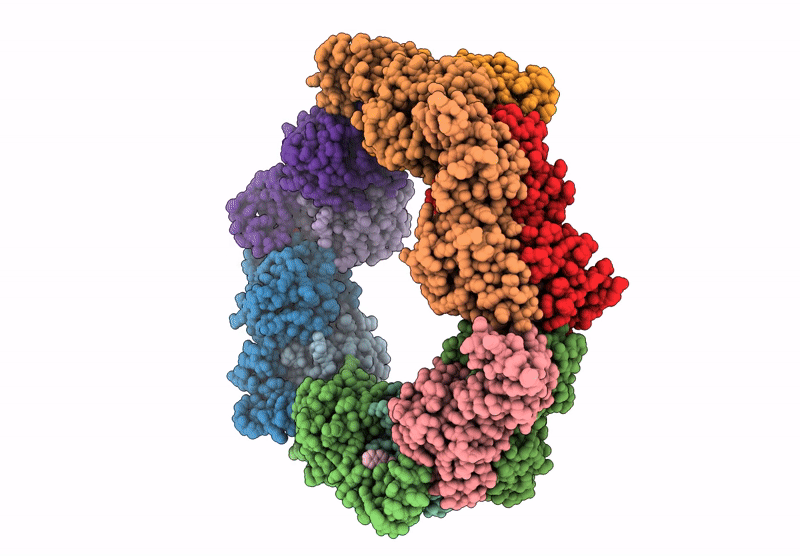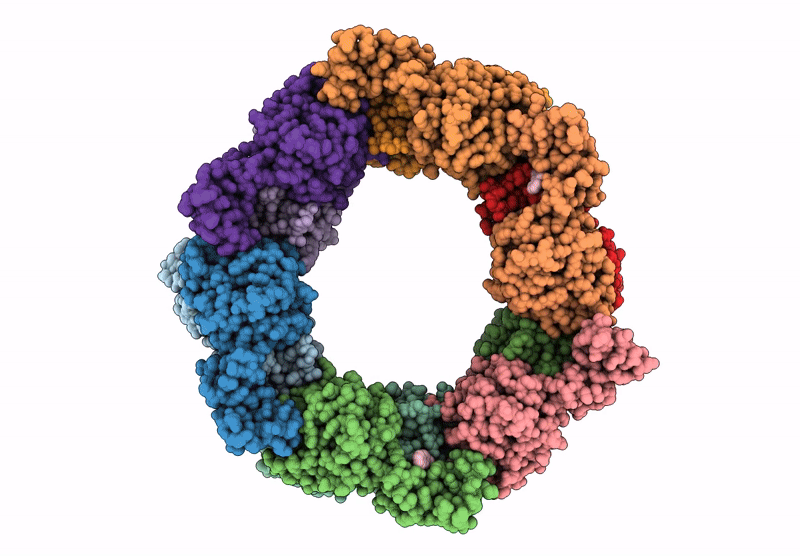
Deposition Date
2022-11-29
Release Date
2023-03-22
Last Version Date
2025-07-02
Entry Detail
PDB ID:
8HLA
Keywords:
Title:
Heteromeric ring comprised of peroxiredoxin from Thermococcus kodakaraensis (TkPrx) F42C/C46S/C205S/C211S mutant modified with 2-(bromoacetyl)naphthalene (Naph@TkPrx*F42C) and TkPrx C46S/F76C/C205S/C211S mutant modified with 2-(bromoacetyl)naphthalene (Naph@TkPrx*F76C) (Naph@(MIX|3:3))
Biological Source:
Source Organism(s):
Thermococcus kodakarensis KOD1 (Taxon ID: 69014)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.81 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE