Deposition Date
2022-11-28
Release Date
2023-10-18
Last Version Date
2024-01-24
Entry Detail
PDB ID:
8HKR
Keywords:
Title:
Crystal Structure of Histone H3 Lysine 79 (H3K79) Methyltransferase Rv2067c from Mycobacterium tuberculosis
Biological Source:
Source Organism:
Mycobacterium tuberculosis H37Rv (Taxon ID: 83332)
Host Organism:
Method Details:
Experimental Method:
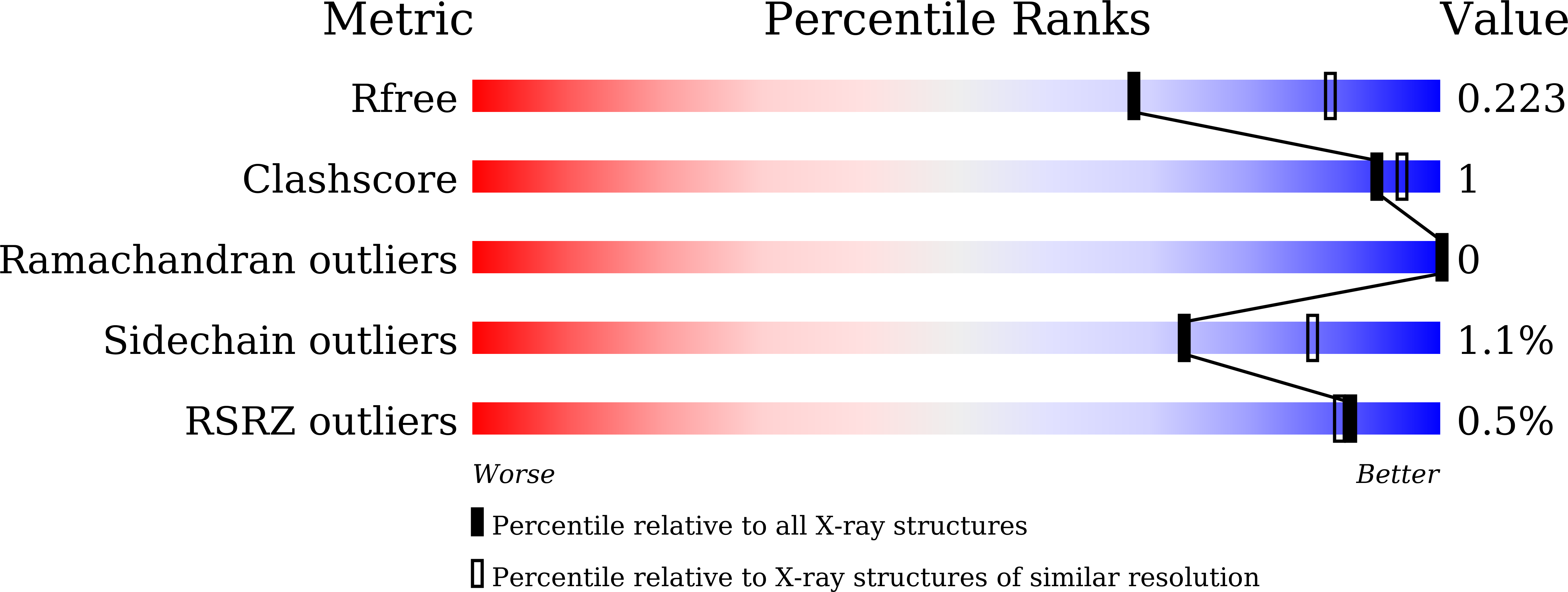
Resolution:
2.40 Å
R-Value Free:
0.21
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 41 21 2