Deposition Date
2022-11-22
Release Date
2023-01-25
Last Version Date
2025-07-02
Entry Detail
PDB ID:
8HJ4
Keywords:
Title:
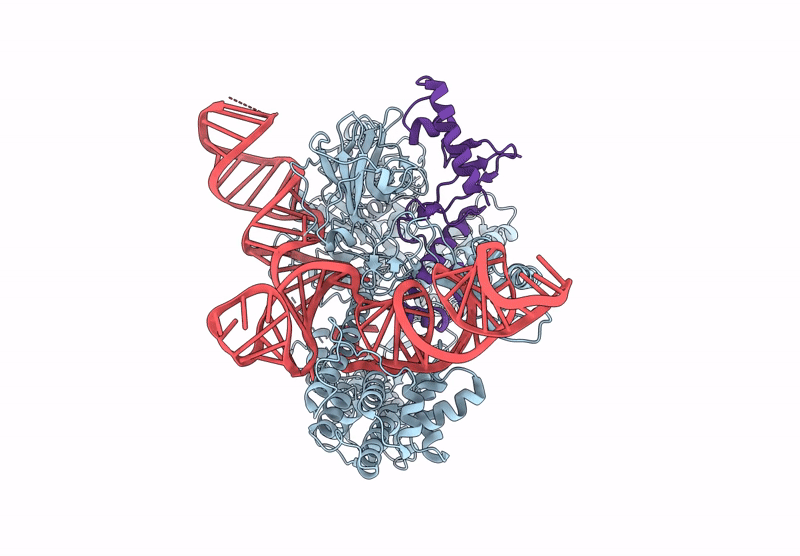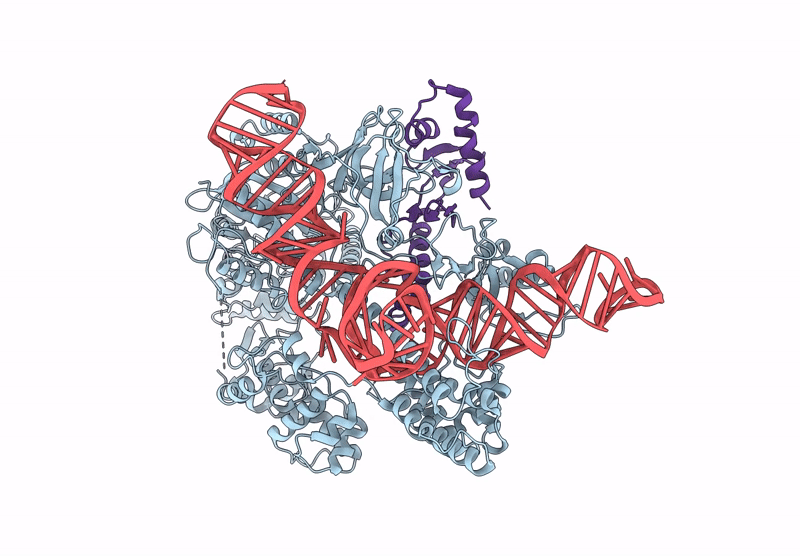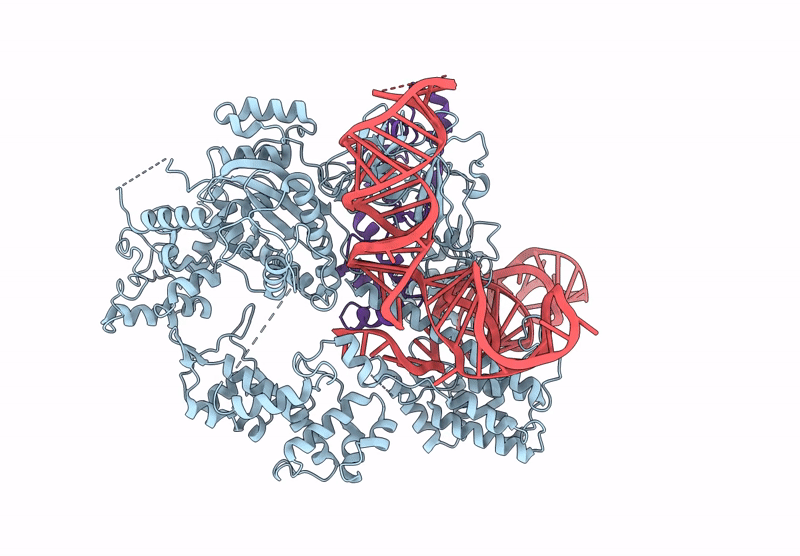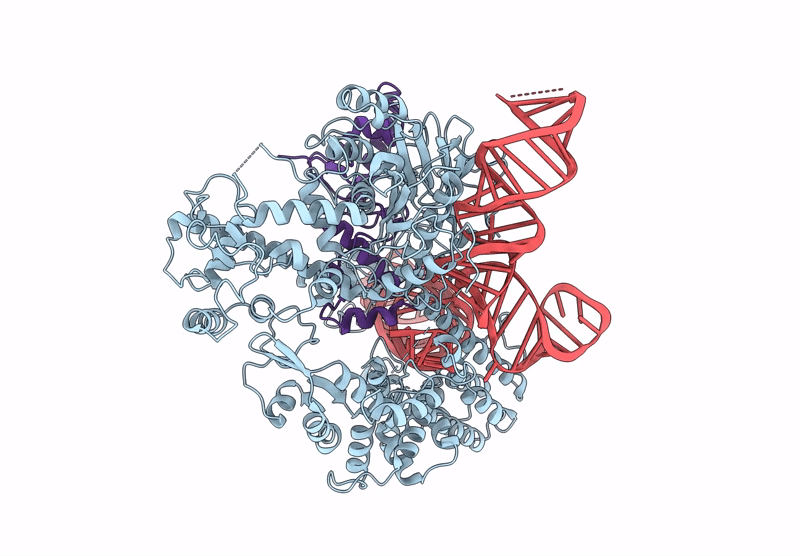
CryoEM structure of an anti-CRISPR protein AcrIIC5 bound to Nme1Cas9-sgRNA complex
Biological Source:
Source Organism(s):
Neisseria meningitidis serogroup C (strain 8013) (Taxon ID: 604162)
Simonsiella muelleri ATCC 29453 (Taxon ID: 641147)
Simonsiella muelleri ATCC 29453 (Taxon ID: 641147)
Expression System(s):
Method Details:
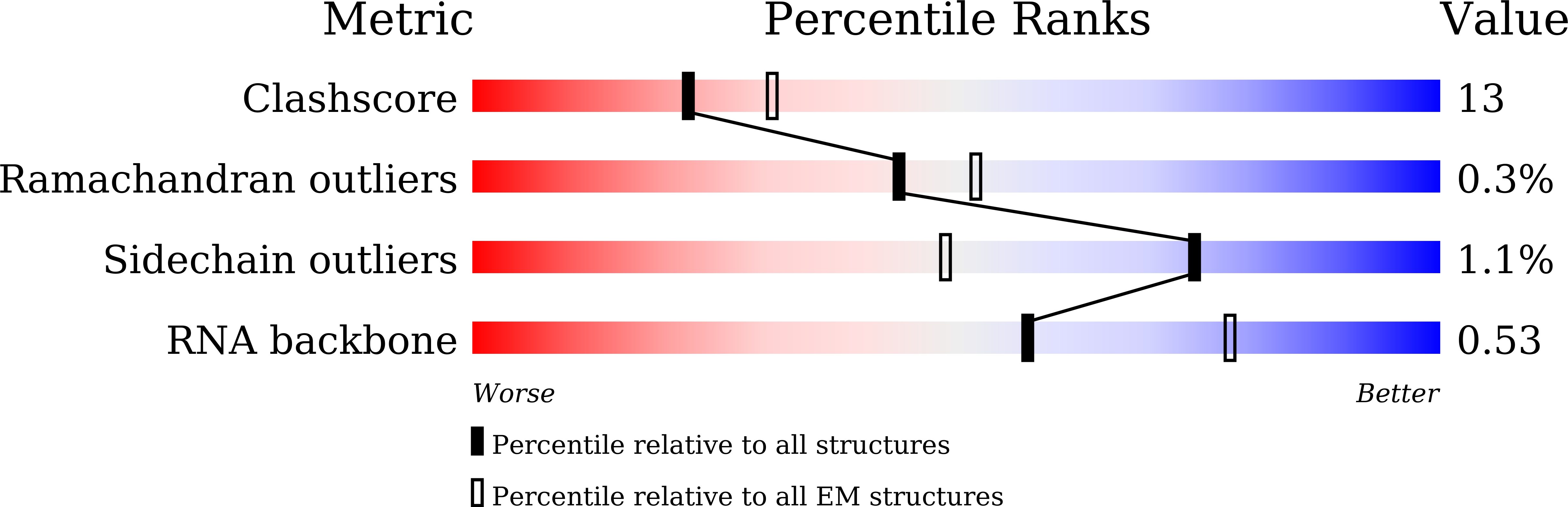
Experimental Method:
Resolution:
3.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE