Deposition Date
2022-10-25
Release Date
2023-07-26
Last Version Date
2024-05-29
Entry Detail
PDB ID:
8H9Y
Keywords:
Title:
Crystal structure of voltage-gated sodium channel NavAb N49K/L176Q mutant in calcium ion condition
Biological Source:
Source Organism(s):
Aliarcobacter butzleri (Taxon ID: 28197)
Expression System(s):
Method Details:
Experimental Method:
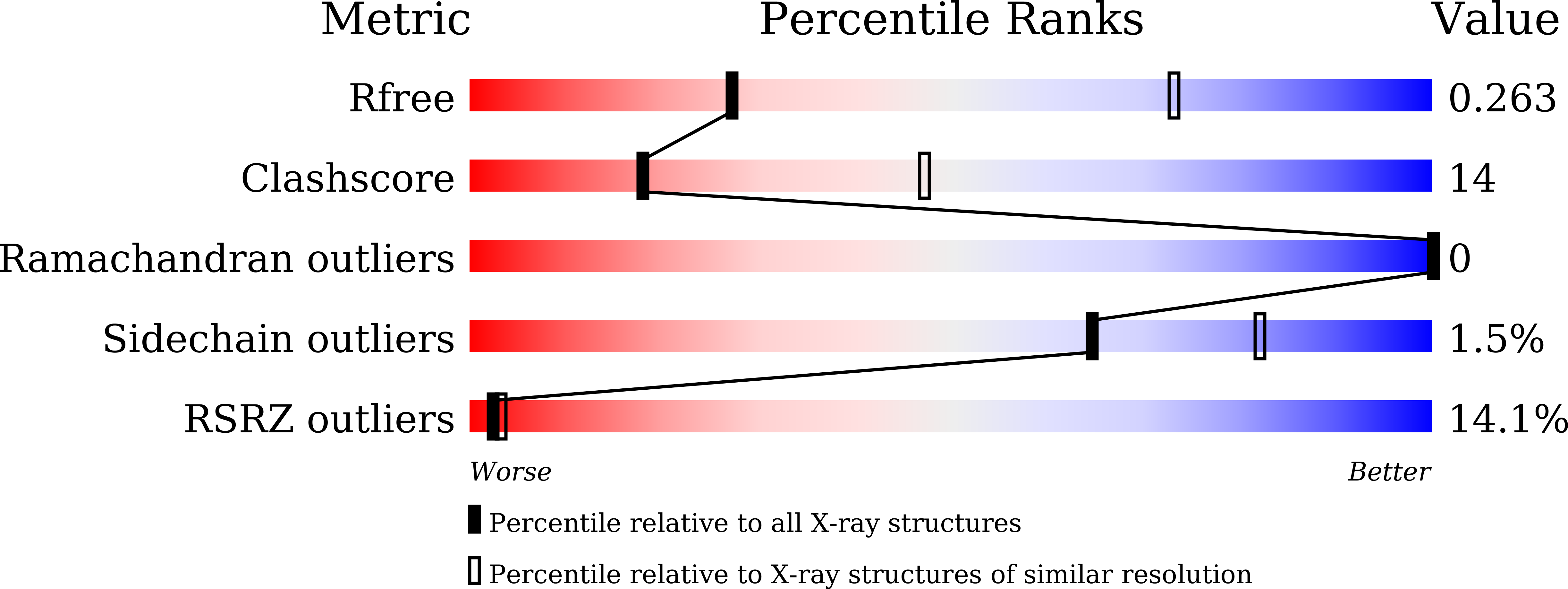
Resolution:
3.40 Å
R-Value Free:
0.26
R-Value Work:
0.25
R-Value Observed:
0.25
Space Group:
I 4 2 2