Deposition Date
2022-08-31
Release Date
2023-01-11
Last Version Date
2024-10-16
Entry Detail
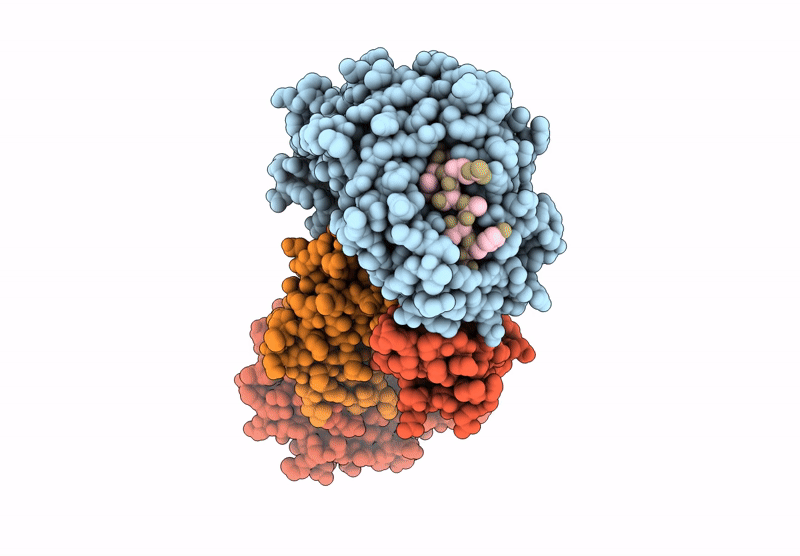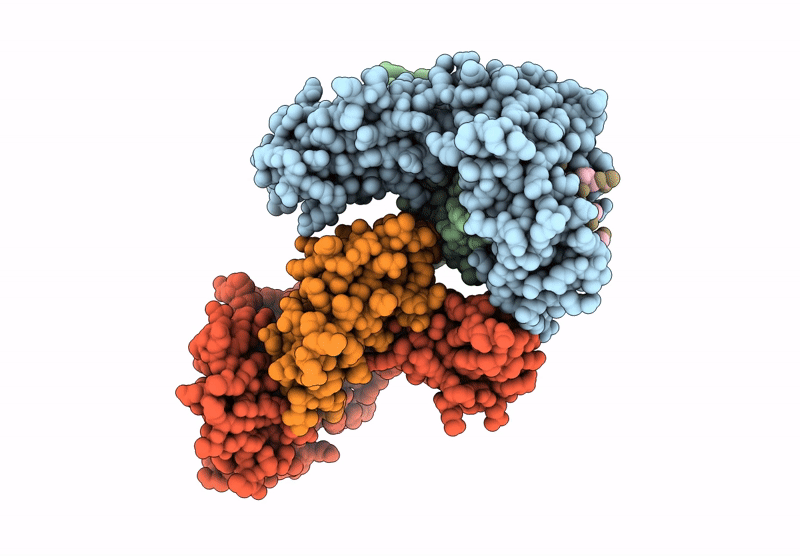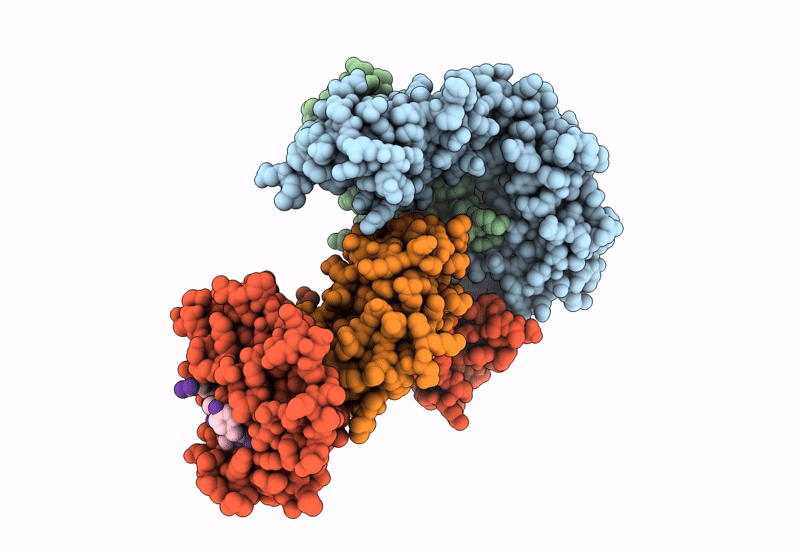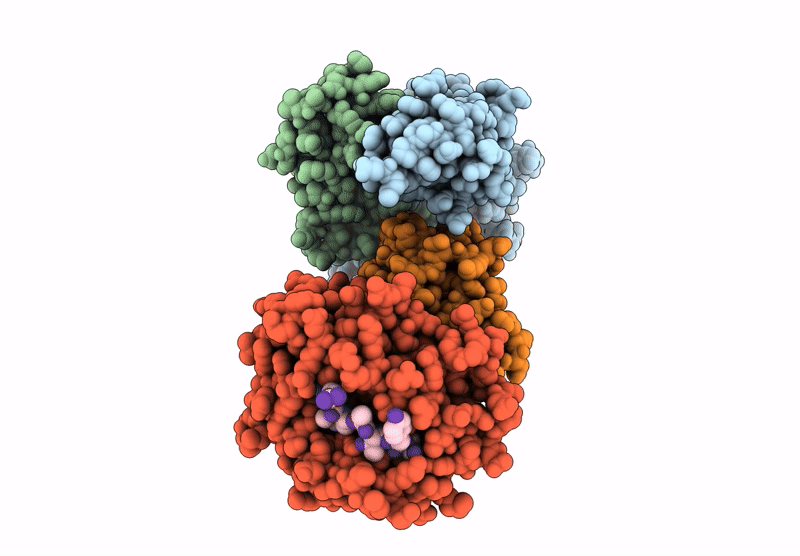
PDB ID:
8GQV
Keywords:
Title:
The Crystal Structures of a Swine SLA-2*HB01 Molecules Complexed with a CTL epitope from Asia1 serotype of Foot-and-mouth disease virus
Biological Source:
Source Organism(s):
Sus scrofa (Taxon ID: 9823)
Foot-and-mouth disease virus (Taxon ID: 12110)
Foot-and-mouth disease virus (Taxon ID: 12110)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.40 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 21 21 21


