Deposition Date
2023-03-15
Release Date
2024-01-17
Last Version Date
2024-01-31
Entry Detail
PDB ID:
8GJD
Keywords:
Title:
X-ray crystallographic structure of a beta-hairpin peptide derived from Abeta 17-36. (ORN)LVFFAED(ORN)AII(N-Me-Gly)LMV
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630 )
Method Details:
Experimental Method:
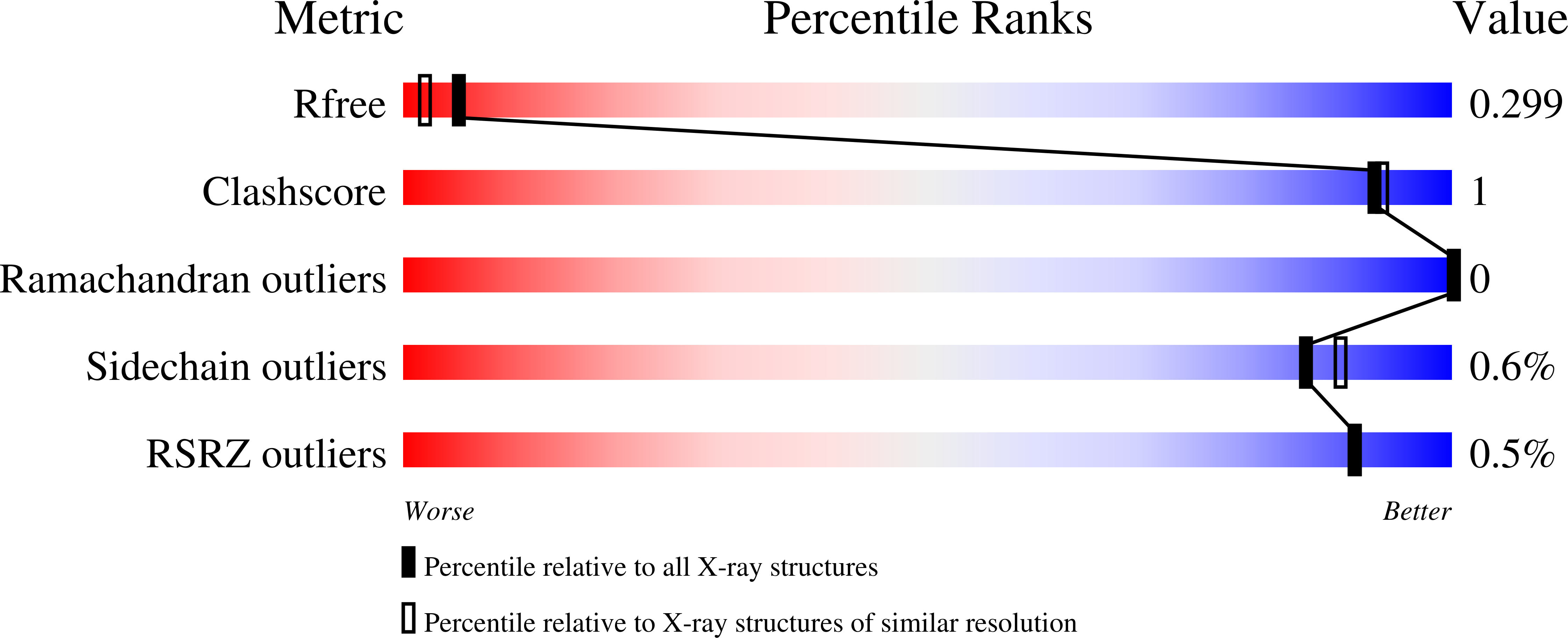
Resolution:
2.03 Å
R-Value Free:
0.29
R-Value Work:
0.23
R-Value Observed:
0.24
Space Group:
H 3