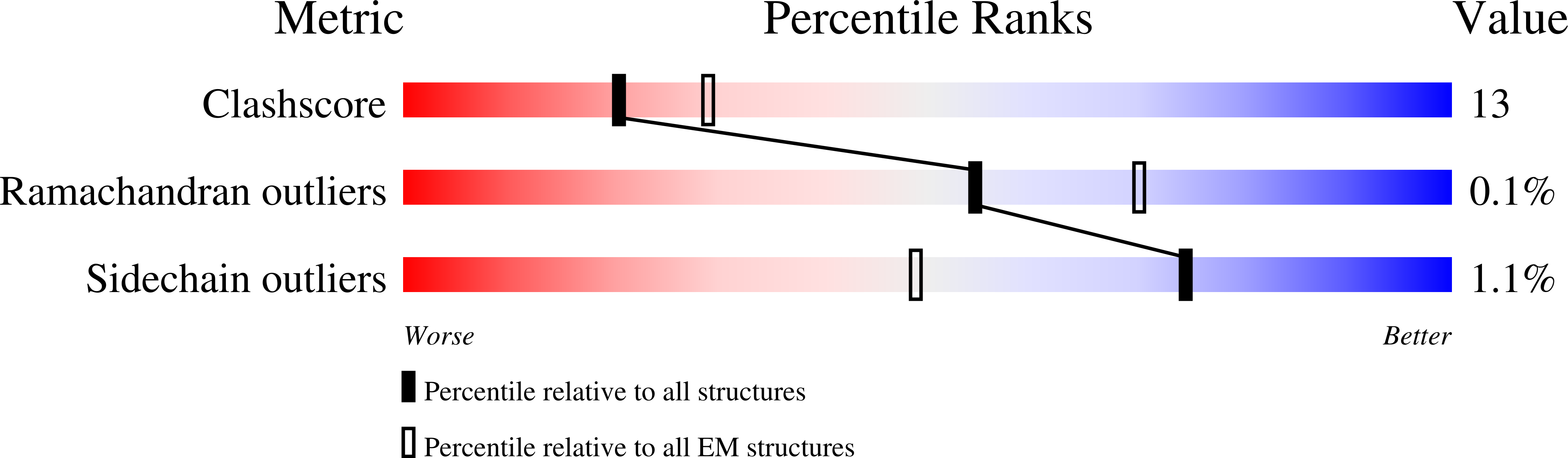
Deposition Date
2023-03-10
Release Date
2023-08-09
Last Version Date
2024-10-16
Entry Detail
PDB ID:
8GHS
Keywords:
Title:
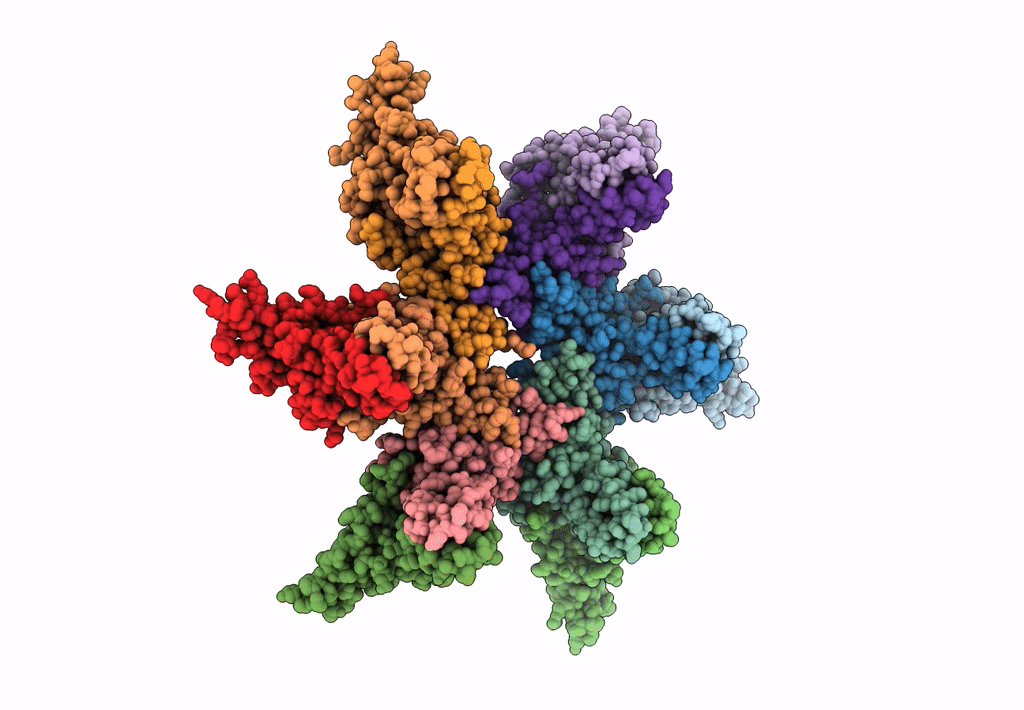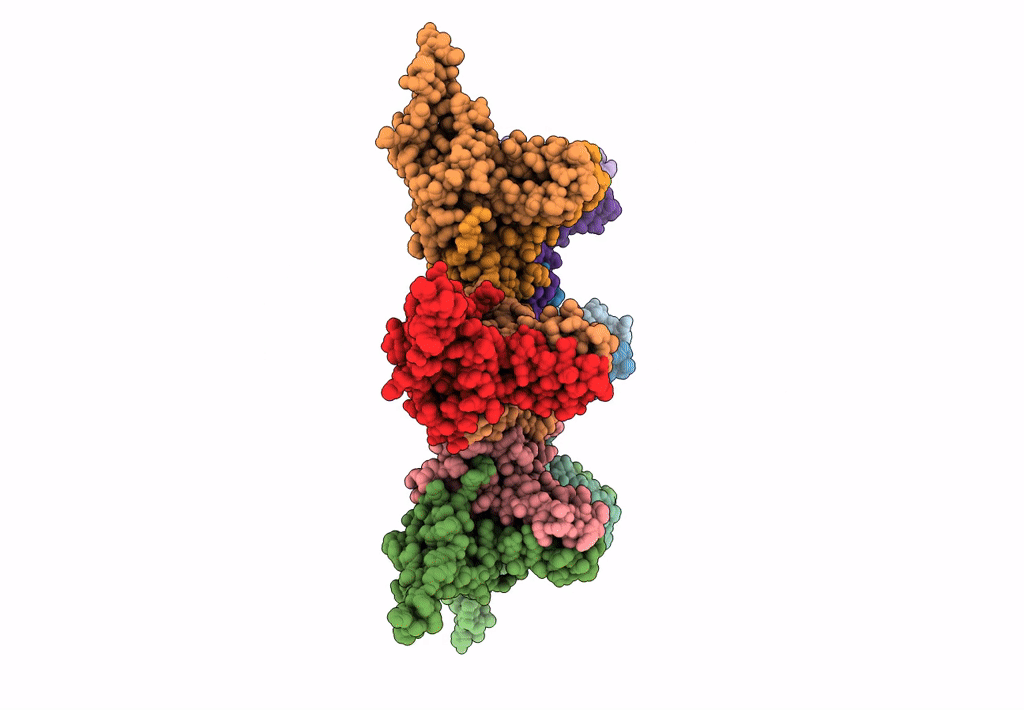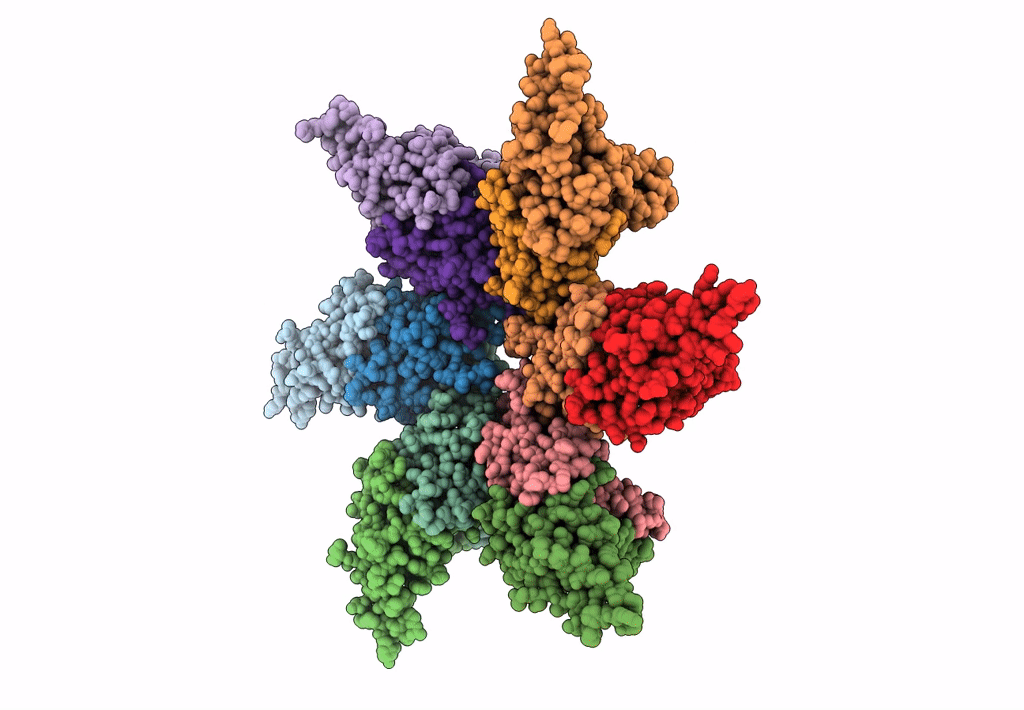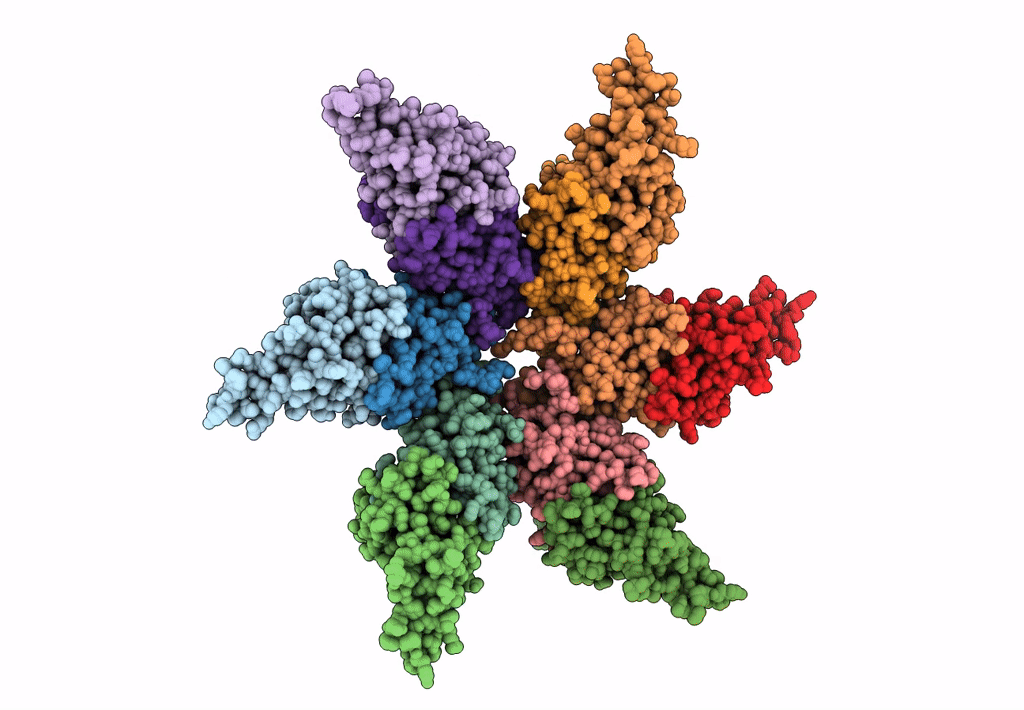
Empty HBV Cp183 capsid with importin-beta, subparticle reconstruction at 2-fold location
Biological Source:
Source Organism(s):
Hepatitis B virus (Taxon ID: 10407)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE