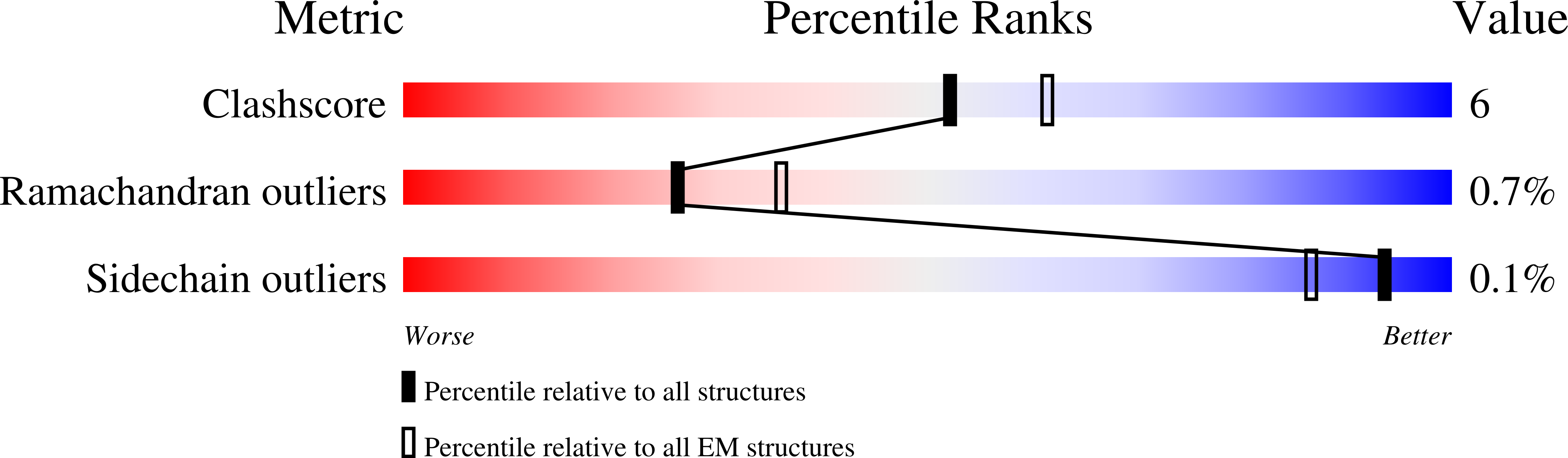Deposition Date
2023-02-02
Release Date
2024-01-17
Last Version Date
2024-10-16
Entry Detail
PDB ID:
8G1R
Keywords:
Title:
A Vibrio cholerae viral satellite enables efficient horizontal transfer by using an external scaffold to assemble hijacked coat proteins into small capsids
Biological Source:
Source Organism(s):
Vibrio phage ICP1_2011_A (Taxon ID: 1296592)
Vibrio cholerae (Taxon ID: 666)
Vibrio cholerae (Taxon ID: 666)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE