Deposition Date
2023-02-01
Release Date
2023-03-01
Last Version Date
2024-10-30
Entry Detail
PDB ID:
8G18
Keywords:
Title:
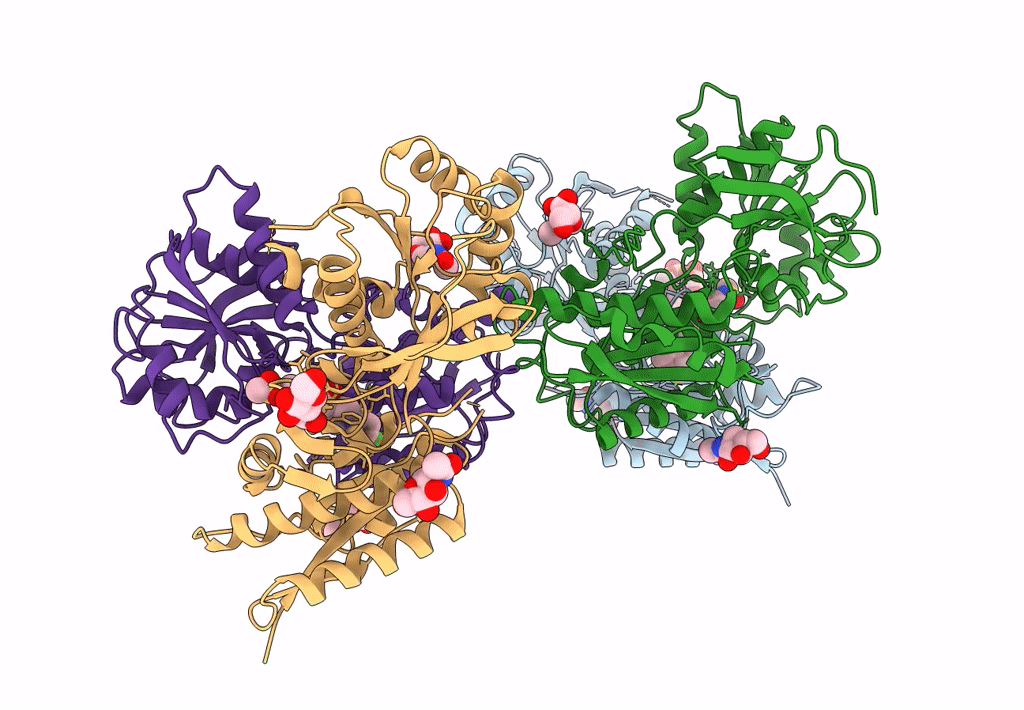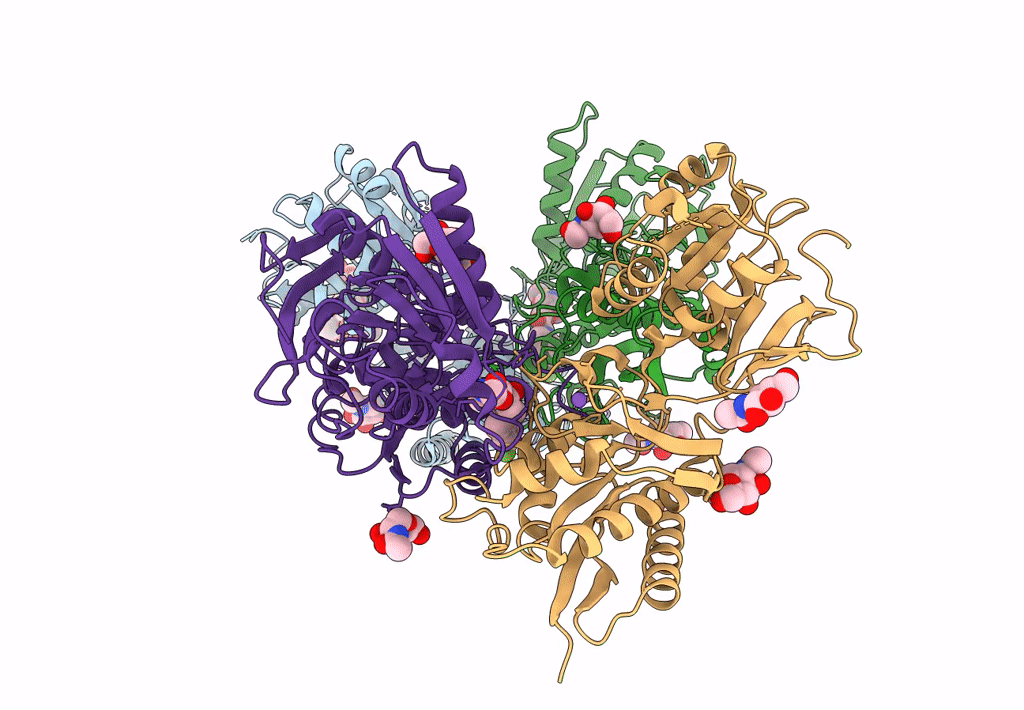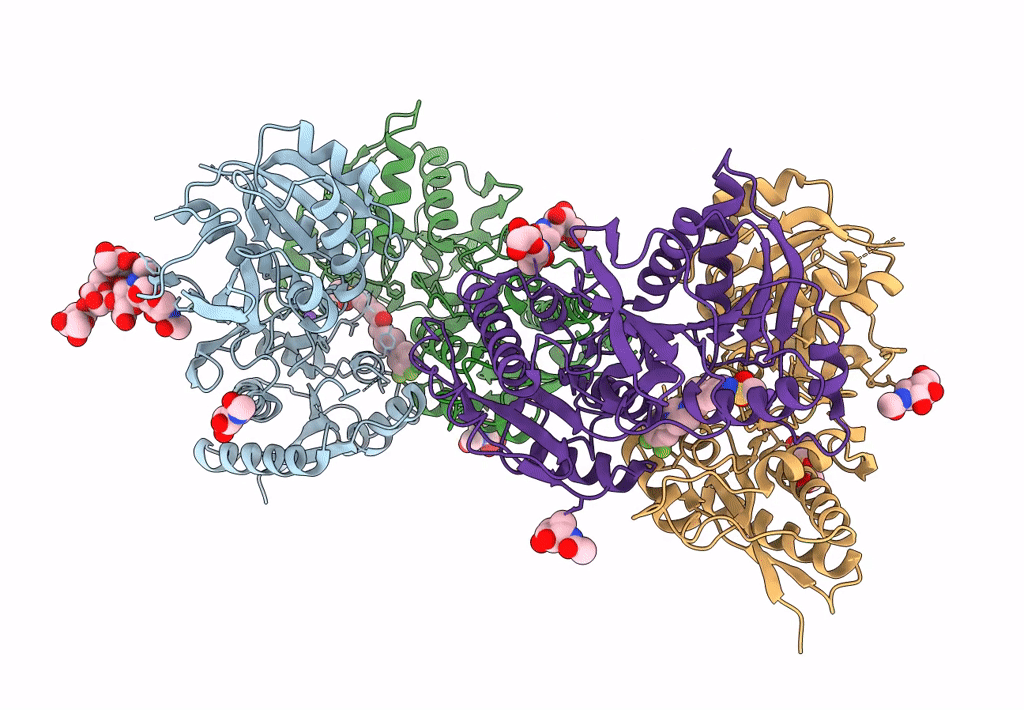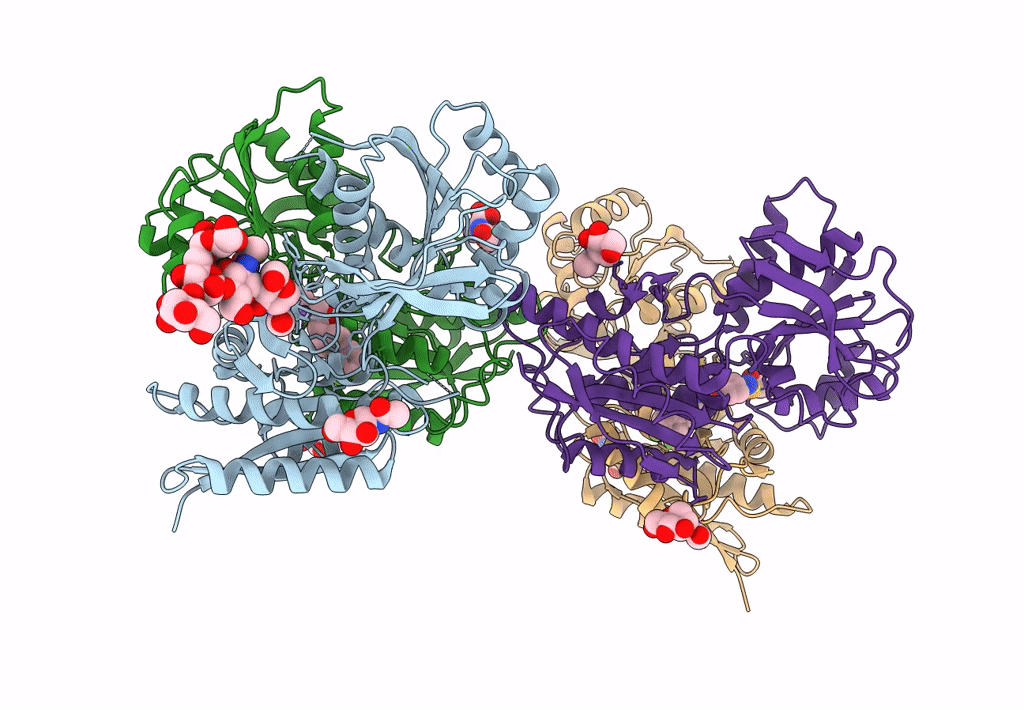
Heterodimer of the GluN1b-GluN2B NMDA receptor amino-terminal domains bound to allosteric inhibitor 93-108
Biological Source:
Source Organism(s):
Xenopus laevis (Taxon ID: 8355)
Rattus norvegicus (Taxon ID: 10116)
Rattus norvegicus (Taxon ID: 10116)
Expression System(s):
Method Details:
Experimental Method:
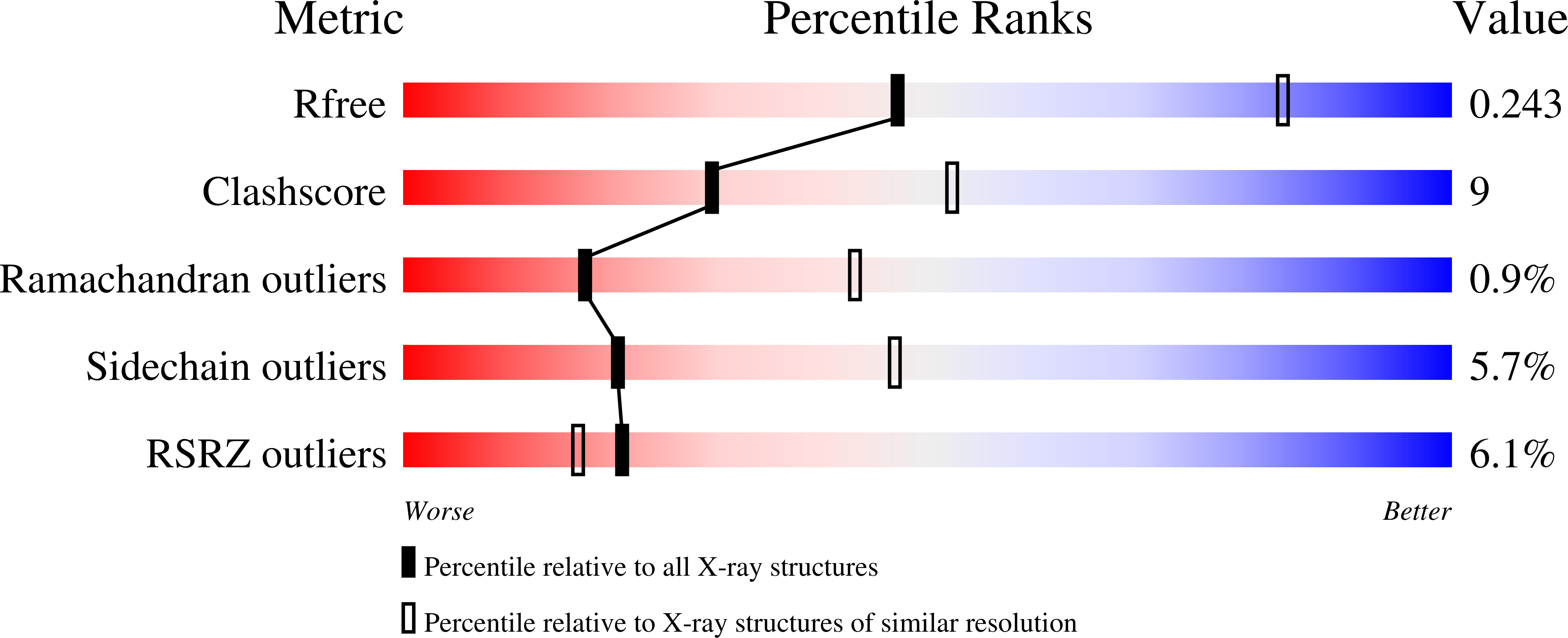
Resolution:
2.85 Å
R-Value Free:
0.24
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1