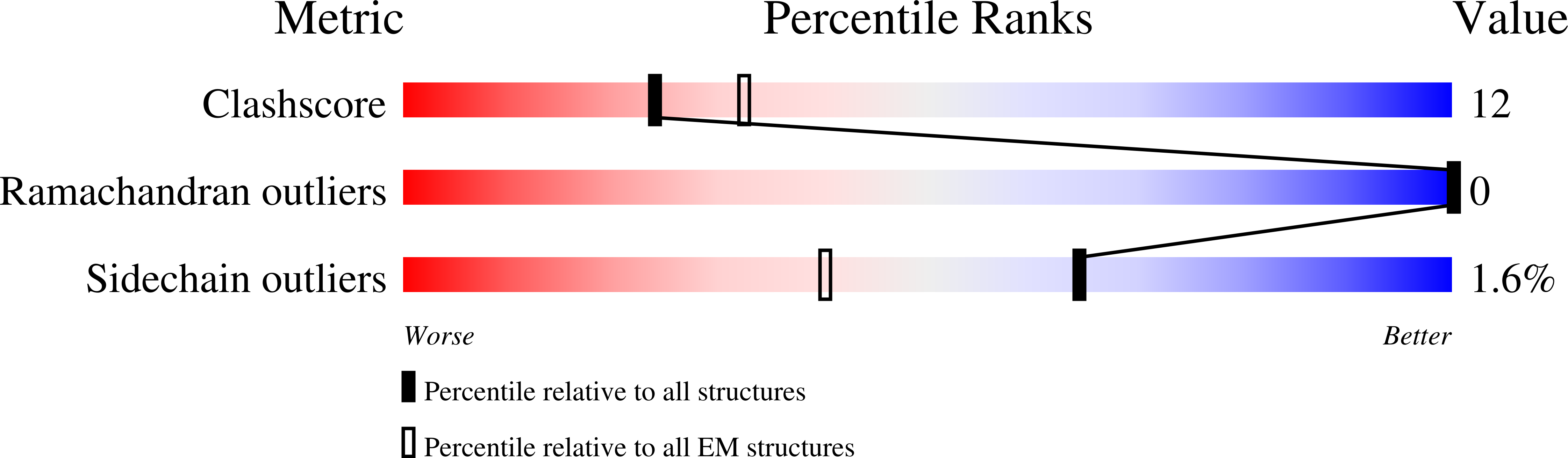
Deposition Date
2023-01-22
Release Date
2023-03-22
Last Version Date
2024-10-23
Entry Detail
PDB ID:
8FWI
Keywords:
Title:
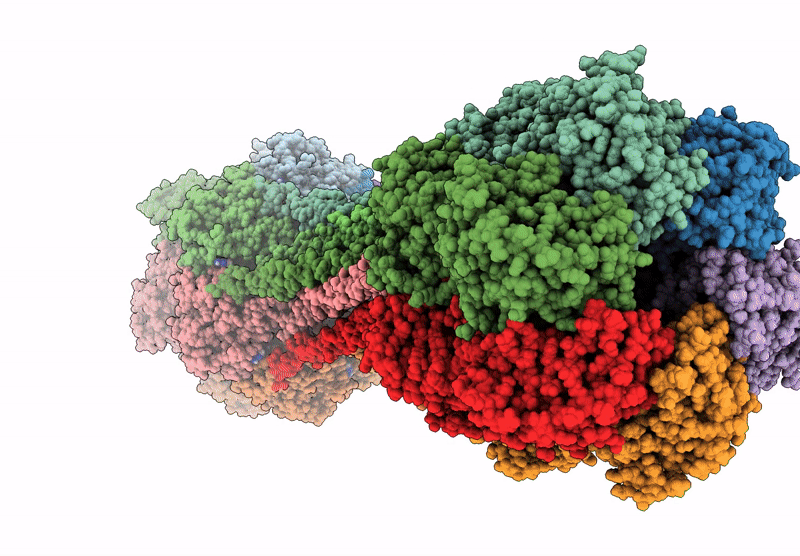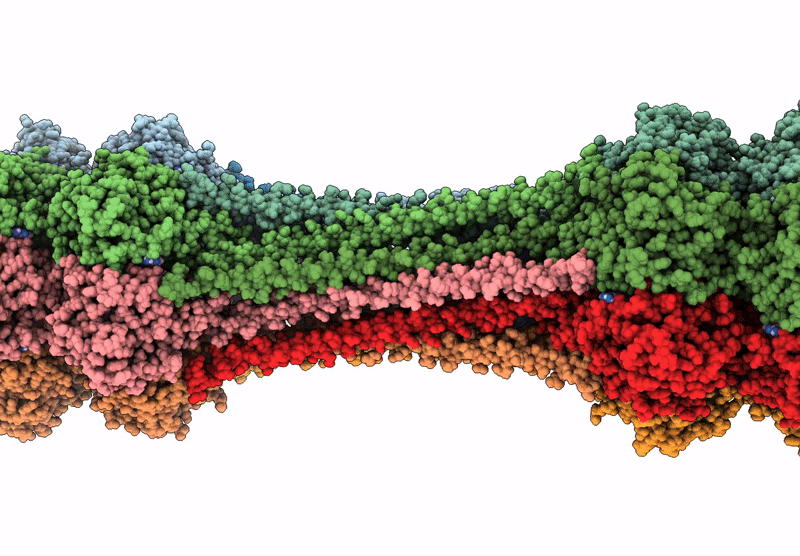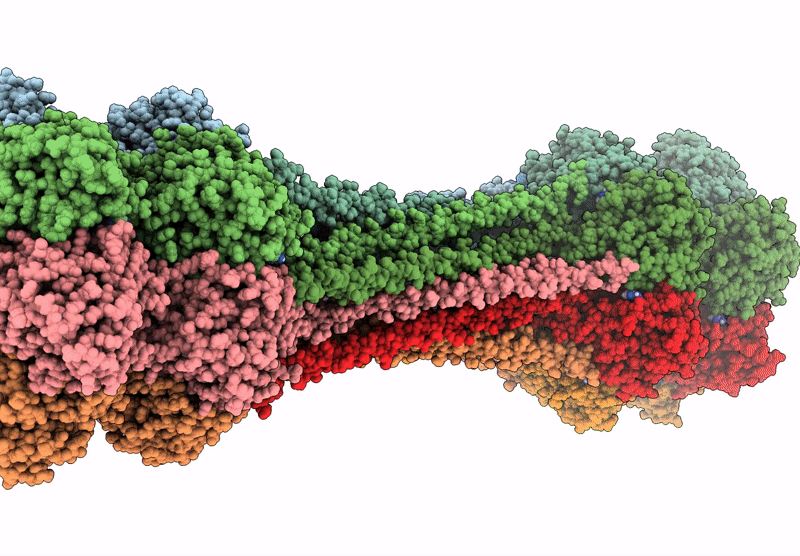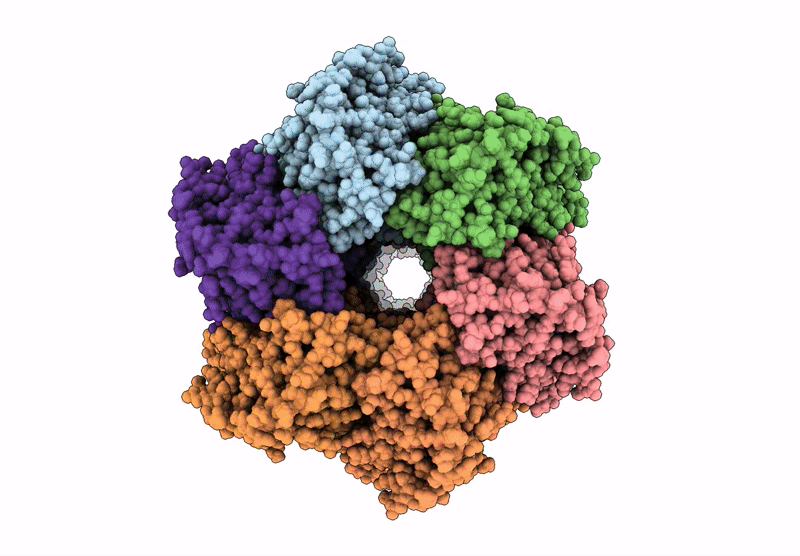
Structure of dodecameric KaiC-RS-S413E/S414E solved by cryo-EM
Biological Source:
Source Organism(s):
Cereibacter sphaeroides (Taxon ID: 557760)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE