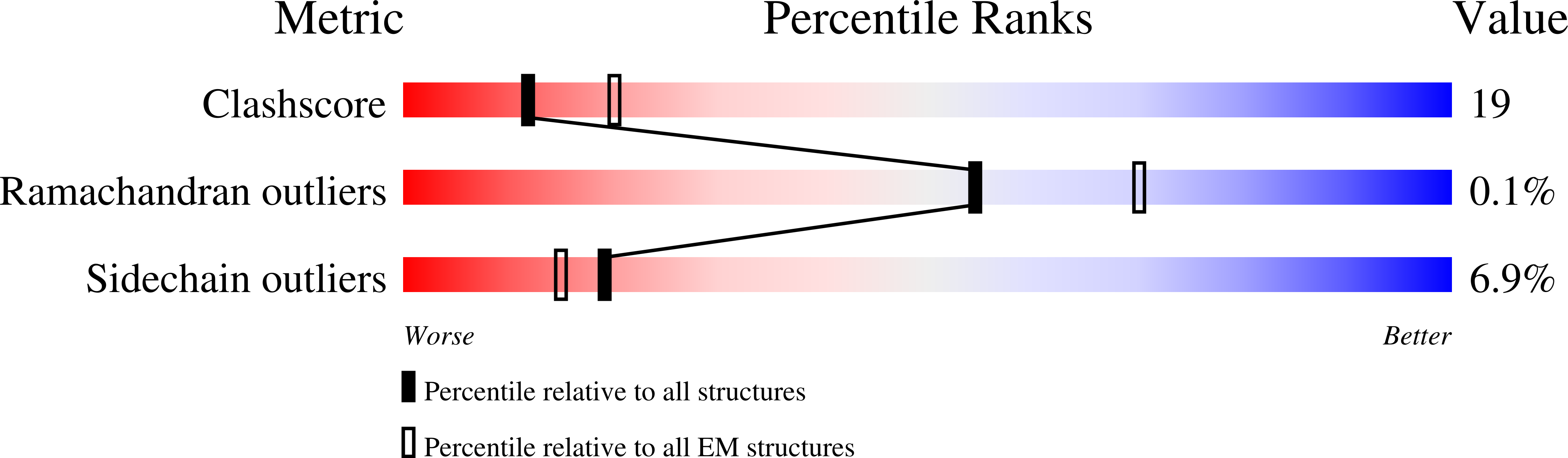Deposition Date
2022-12-23
Release Date
2024-01-10
Last Version Date
2024-11-20
Entry Detail
PDB ID:
8FML
Keywords:
Title:
Cryo-EM structure of NLR family apoptosis inhibitory protein 5 (NAIP5) in complex with a full-length flagellin (FliC) ligand
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (Taxon ID: 99287)
Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (Taxon ID: 99287)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.93 Å
Aggregation State:
CELL
Reconstruction Method:
SINGLE PARTICLE