Deposition Date
2022-12-23
Release Date
2024-02-07
Last Version Date
2024-05-29
Entry Detail
PDB ID:
8FMG
Keywords:
Title:
Structure of CBASS Cap5 from Pseudomonas syringae as an activated tetramer with the cyclic dinucleotide 3'2'-c-diAMP ligand (3 tetramers in the AU)
Biological Source:
Source Organism(s):
Pseudomonas syringae (Taxon ID: 317)
Expression System(s):
Method Details:
Experimental Method:
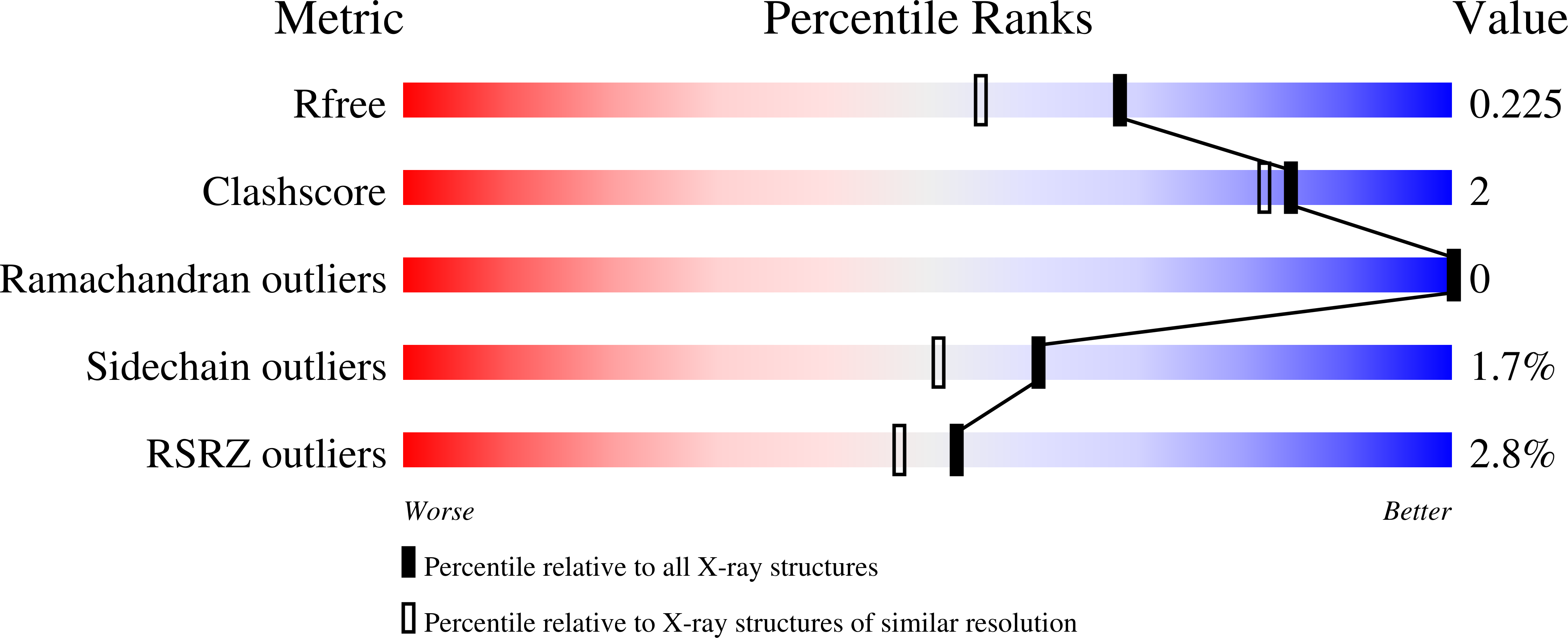
Resolution:
1.79 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
C 1 2 1