Deposition Date
2022-12-04
Release Date
2023-06-21
Last Version Date
2023-11-15
Entry Detail
PDB ID:
8FDU
Keywords:
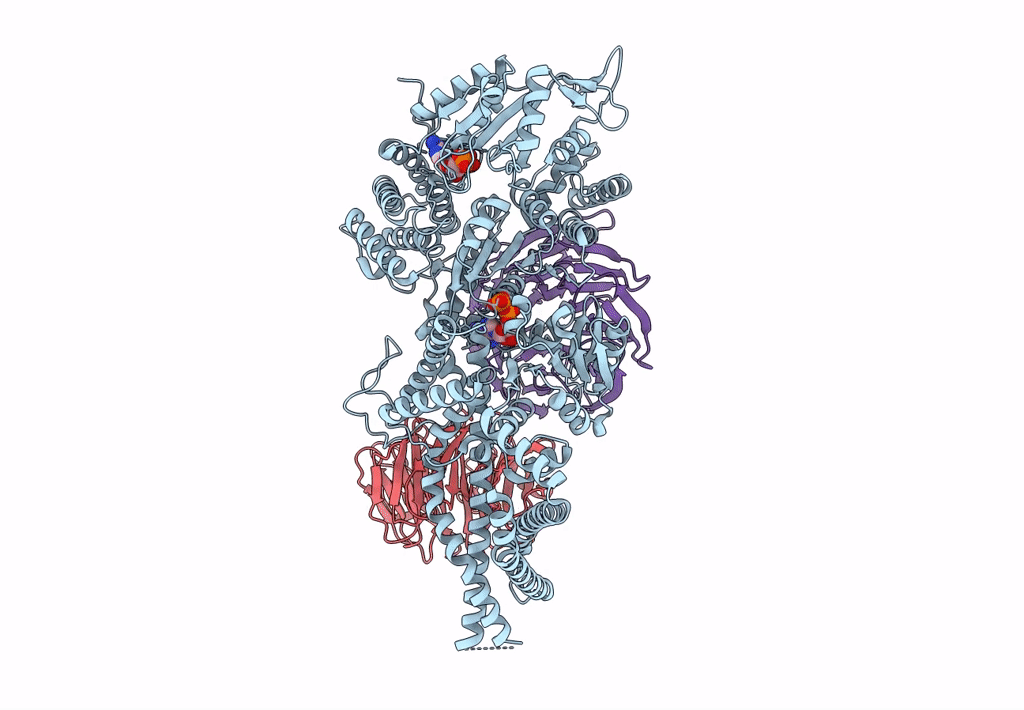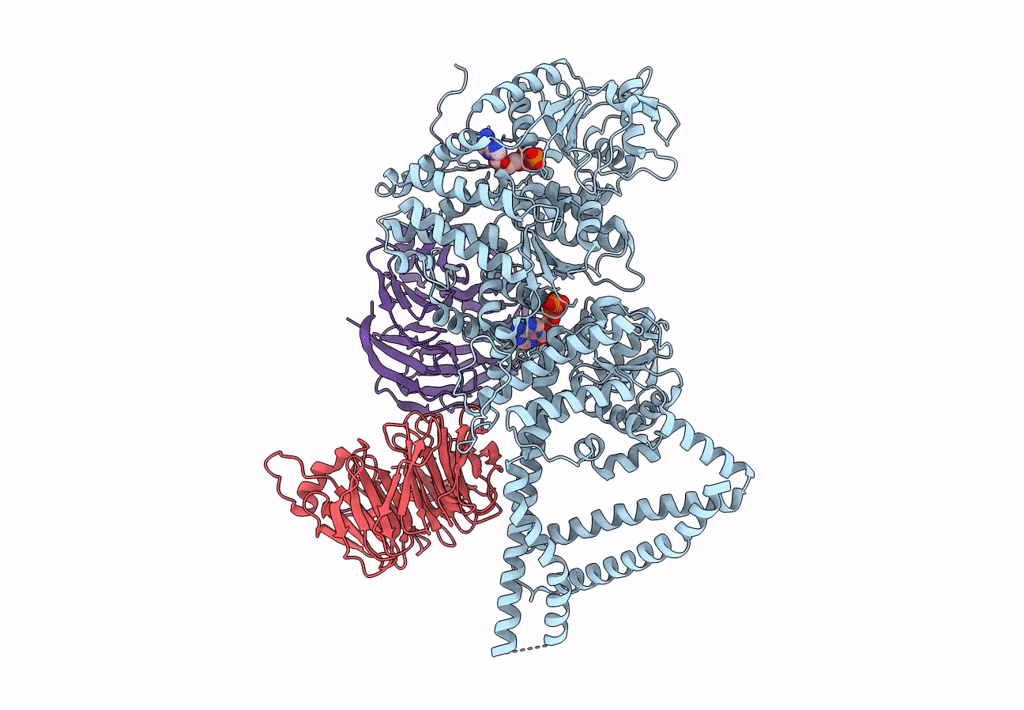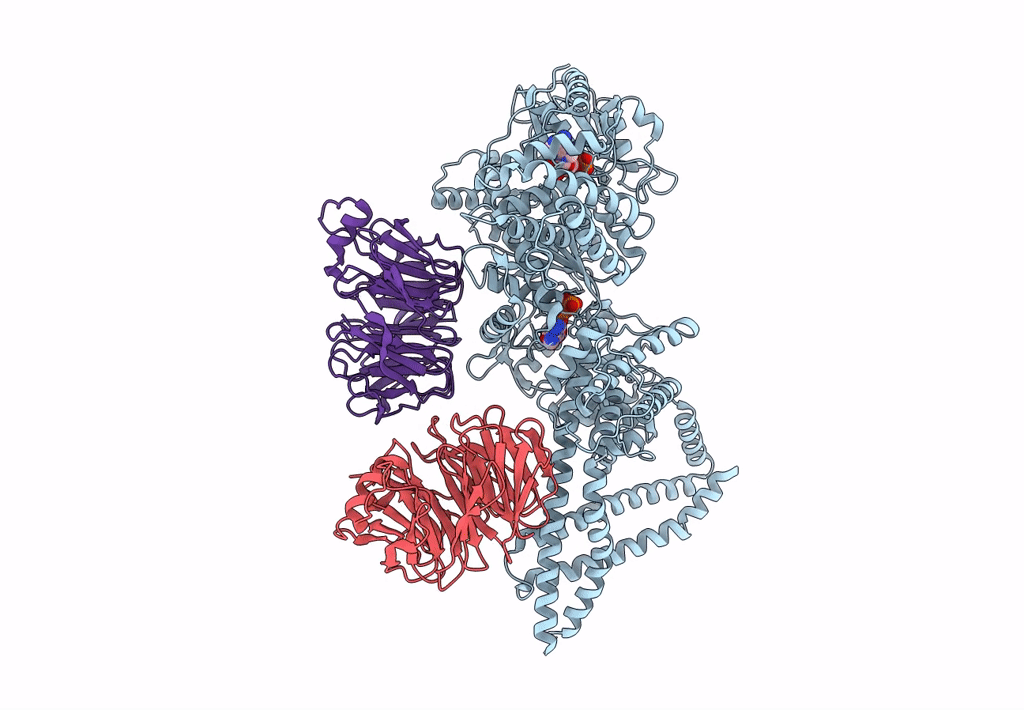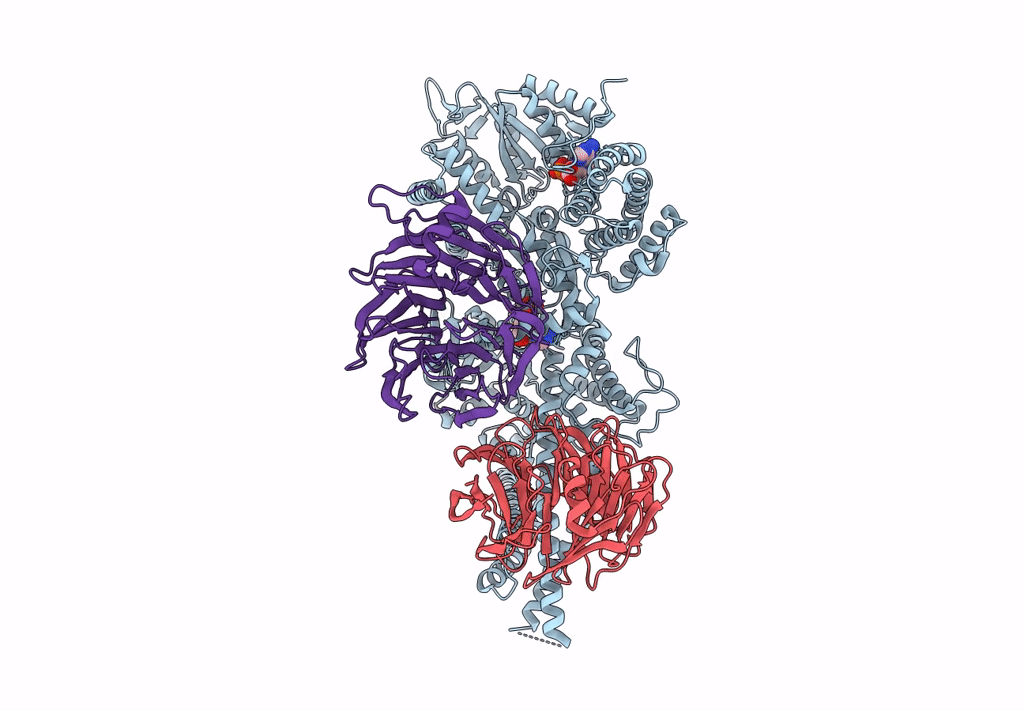
Title:
Engineered human dynein motor domain in the microtubule-unbound state with LIS1 complex in the buffer containing ATP-Vi (local refined on AAA3-AAA5 and LIS1)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Thermus thermophilus (Taxon ID: 300852)
Thermus thermophilus (Taxon ID: 300852)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


