Abstact
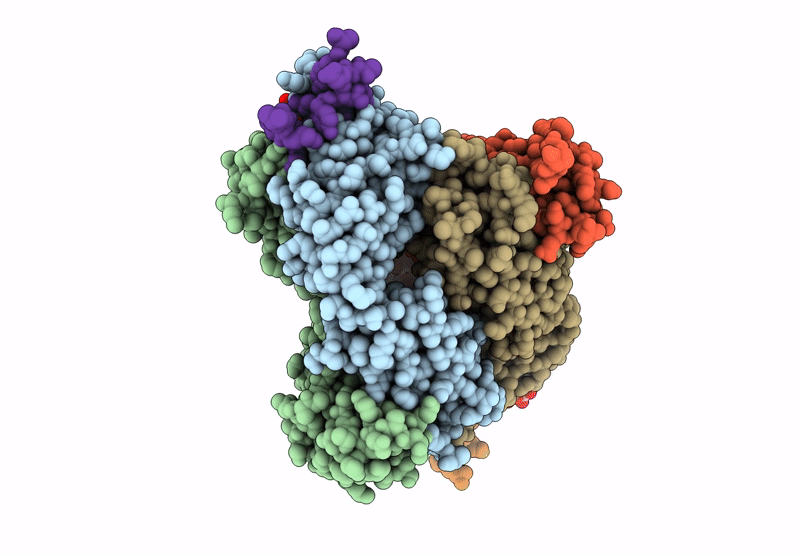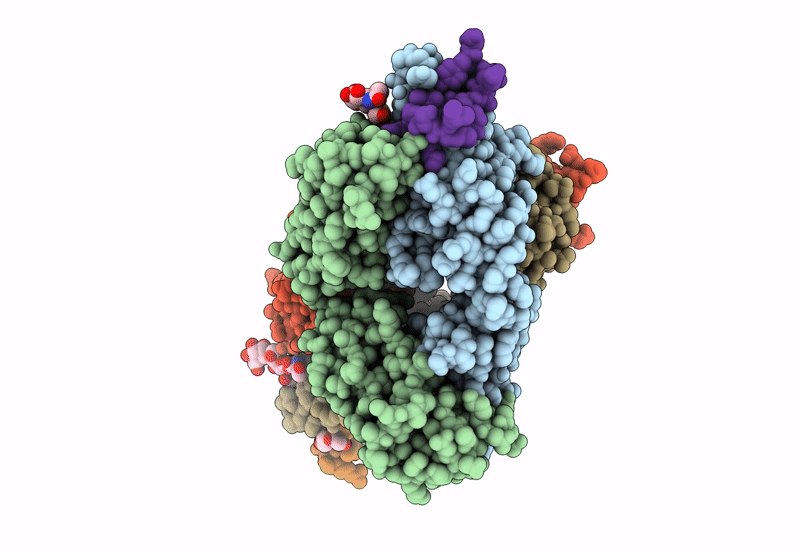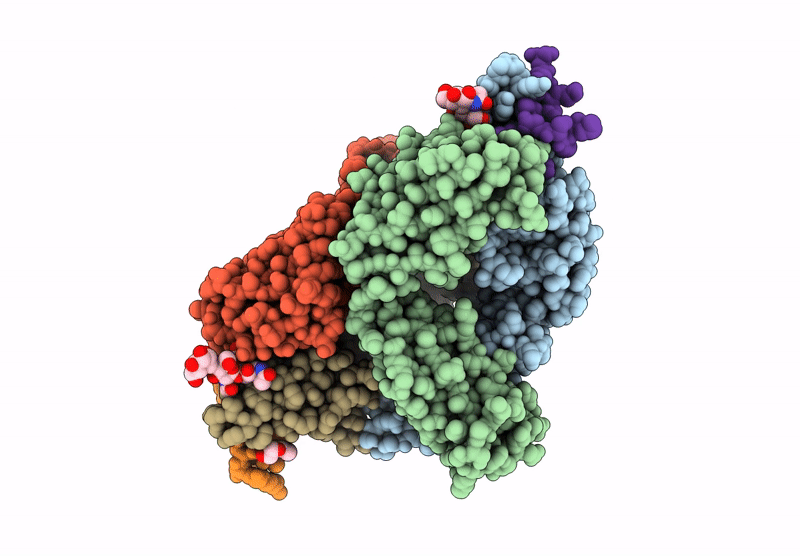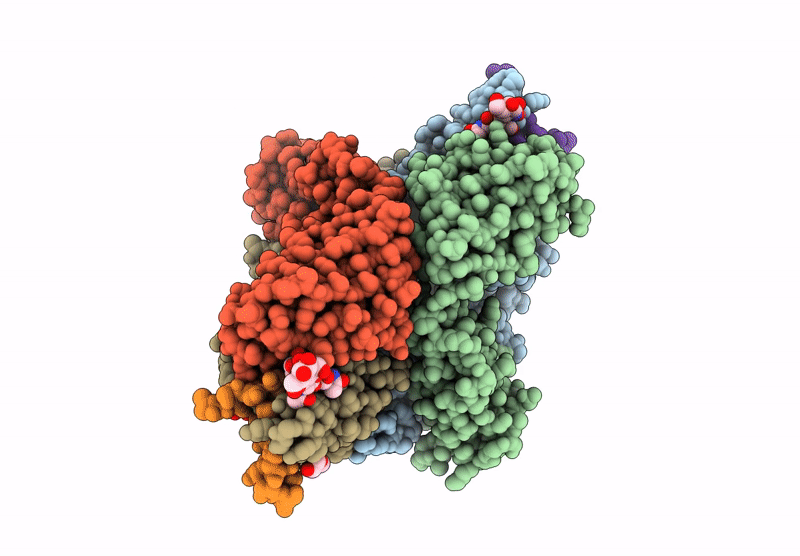
The monoclonal antibodies (MAbs) NCI05 and NCI09, isolated from a vaccinated macaque that was protected from multiple simian immunodeficiency virus (SIV) challenges, both target an overlapping, conformationally dynamic epitope in SIV envelope variable region 2 (V2). Here, we show that NCI05 recognizes a CH59-like coil/helical epitope, whereas NCI09 recognizes a β-hairpin linear epitope. In vitro, NCI05 and, to a lesser extent, NCI09 mediate the killing of SIV-infected cells in a CD4-dependent manner. Compared to NCI05, NCI09 mediates higher titers of antibody-dependent cellular cytotoxicity (ADCC) to gp120-coated cells, as well as higher levels of trogocytosis, a monocyte function that contributes to immune evasion. We also found that passive administration of NCI05 or NCI09 to macaques did not affect the risk of SIVmac251 acquisition compared to controls, demonstrating that these anti-V2 antibodies alone are not protective. However, NCI05 but not NCI09 mucosal levels strongly correlated with delayed SIVmac251 acquisition, and functional and structural data suggest that NCI05 targets a transient state of the viral spike apex that is partially opened, compared to its prefusion-closed conformation. IMPORTANCE Studies suggest that the protection against SIV/simian-human immunodeficiency virus (SHIV) acquisition afforded by the SIV/HIV V1 deletion-containing envelope immunogens, delivered by the DNA/ALVAC vaccine platform, requires multiple innate and adaptive host responses. Anti-inflammatory macrophages and tolerogenic dendritic cells (DC-10), together with CD14+ efferocytes, are consistently found to correlate with a vaccine-induced decrease in the risk of SIV/SHIV acquisition. Similarly, V2-specific antibody responses mediating ADCC, Th1 and Th2 cells expressing no or low levels of CCR5, and envelope-specific NKp44+ cells producing interleukin 17 (IL-17) also are reproducible correlates of decreased risk of virus acquisition. We focused on the function and the antiviral potential of two monoclonal antibodies (NCI05 and NCI09) isolated from vaccinated animals that differ in antiviral function in vitro and recognize V2 in a linear (NCI09) or coil/helical (NCI05) conformation. We demonstrate that NCI05, but not NCI09, delays SIVmac251 acquisition, highlighting the complexity of antibody responses to V2.