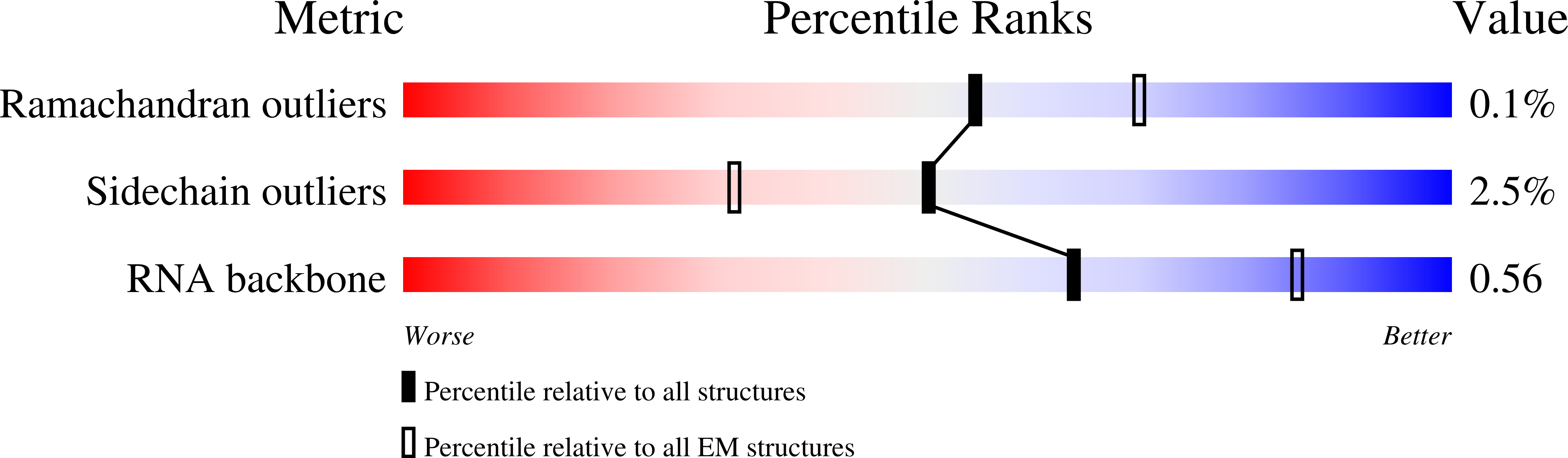
Deposition Date
2022-09-28
Release Date
2023-05-31
Last Version Date
2025-02-12
Entry Detail
PDB ID:
8EMM
Keywords:
Title:
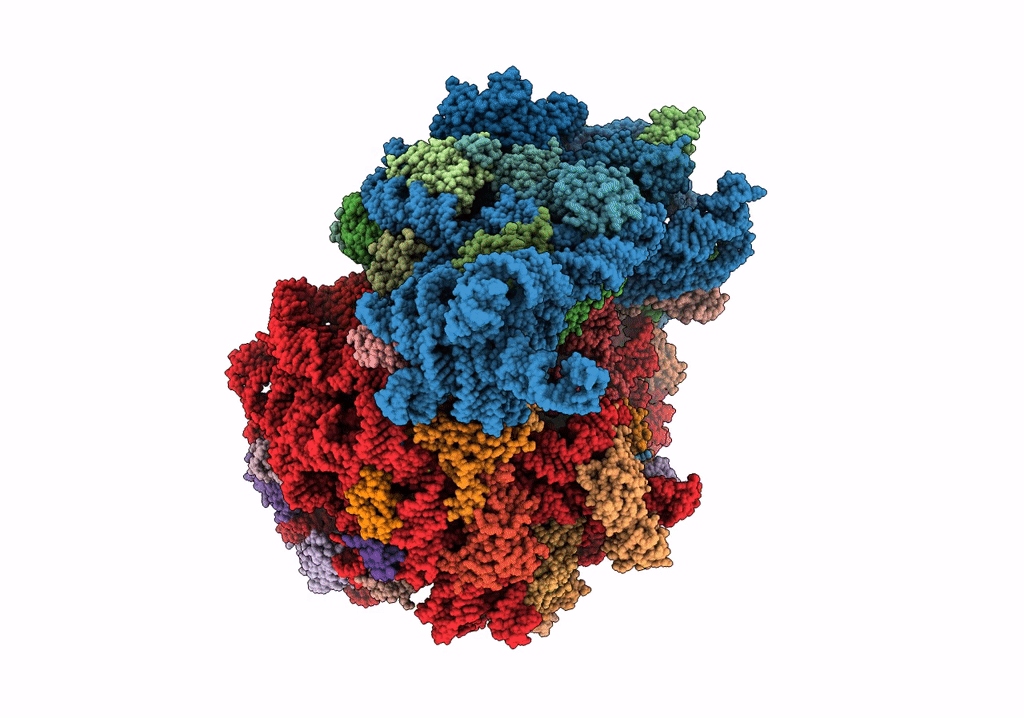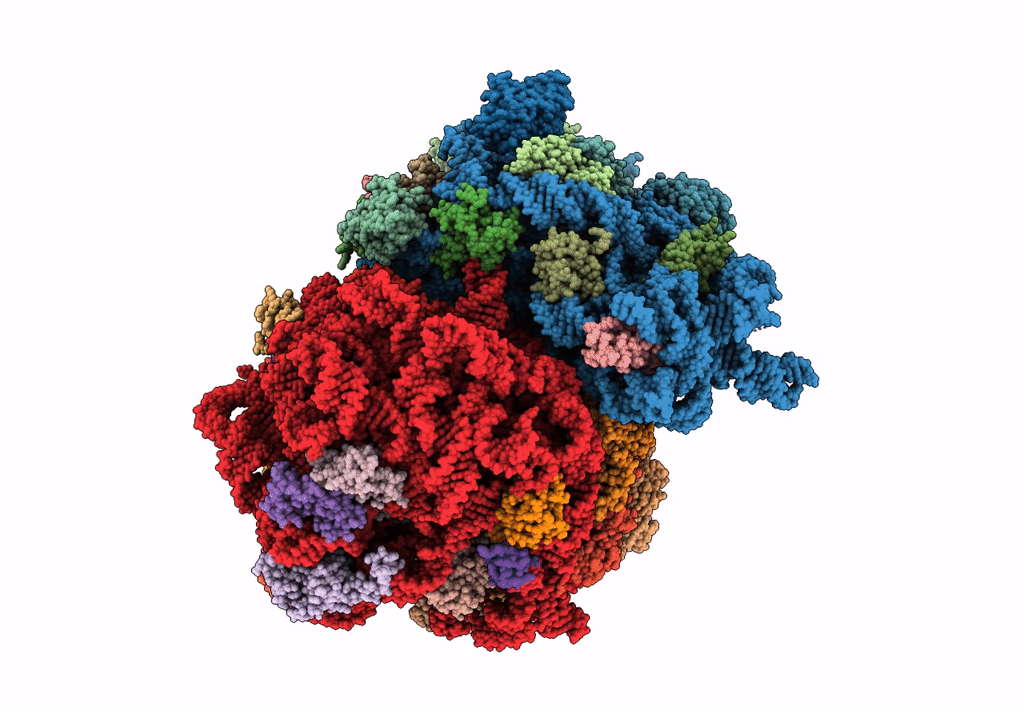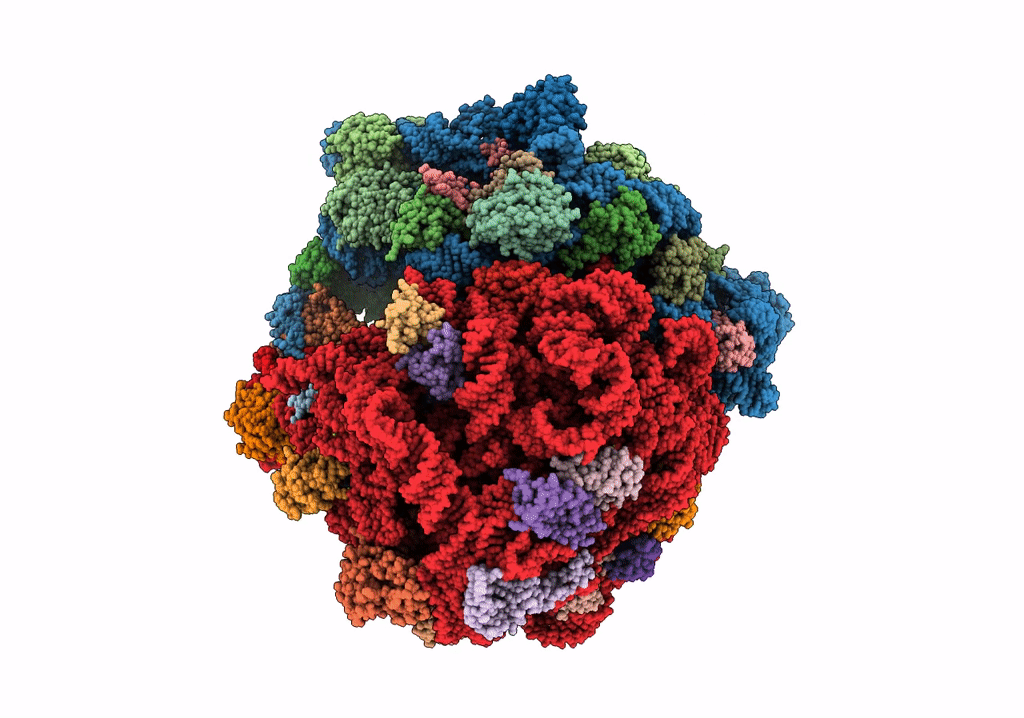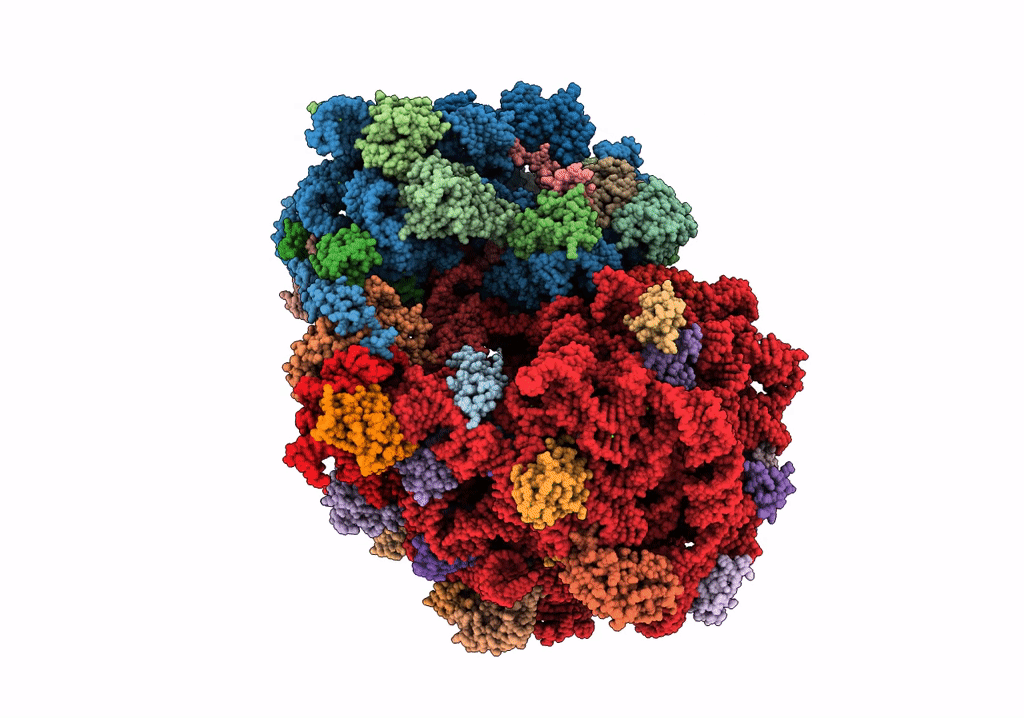
Composite 70S ribosome structure for "Atomistic simulations of the E. coli ribosome provide selection criteria for translationally active substrates
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 562)
Method Details:
Experimental Method:
Resolution:
2.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE