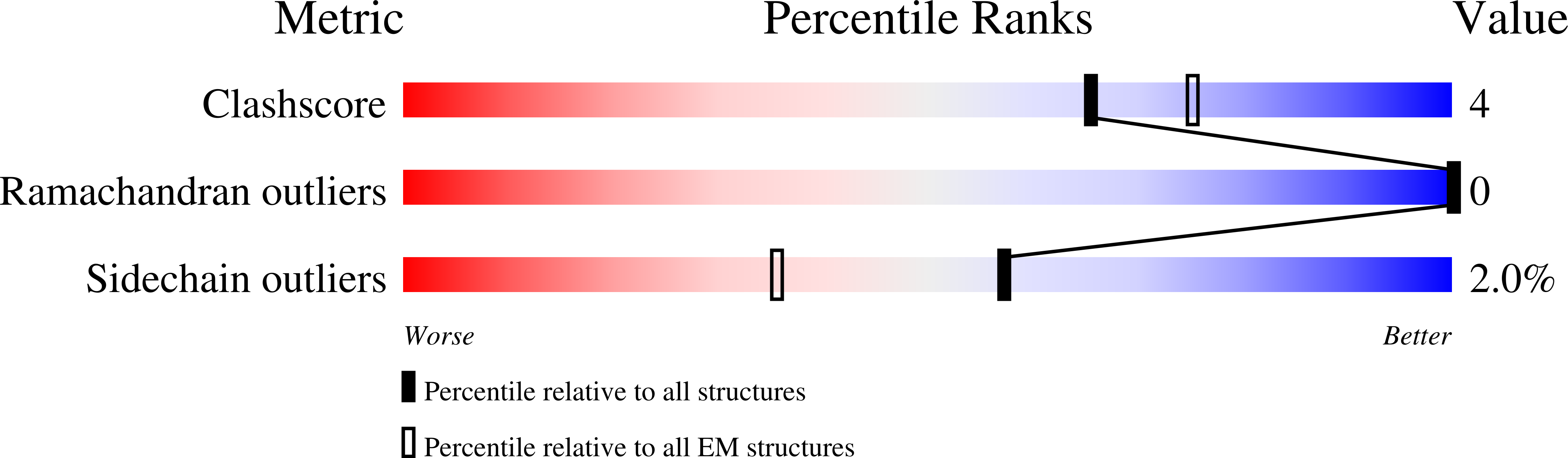
Deposition Date
2022-08-29
Release Date
2022-12-14
Last Version Date
2024-06-19
Entry Detail
PDB ID:
8EAJ
Keywords:
Title:
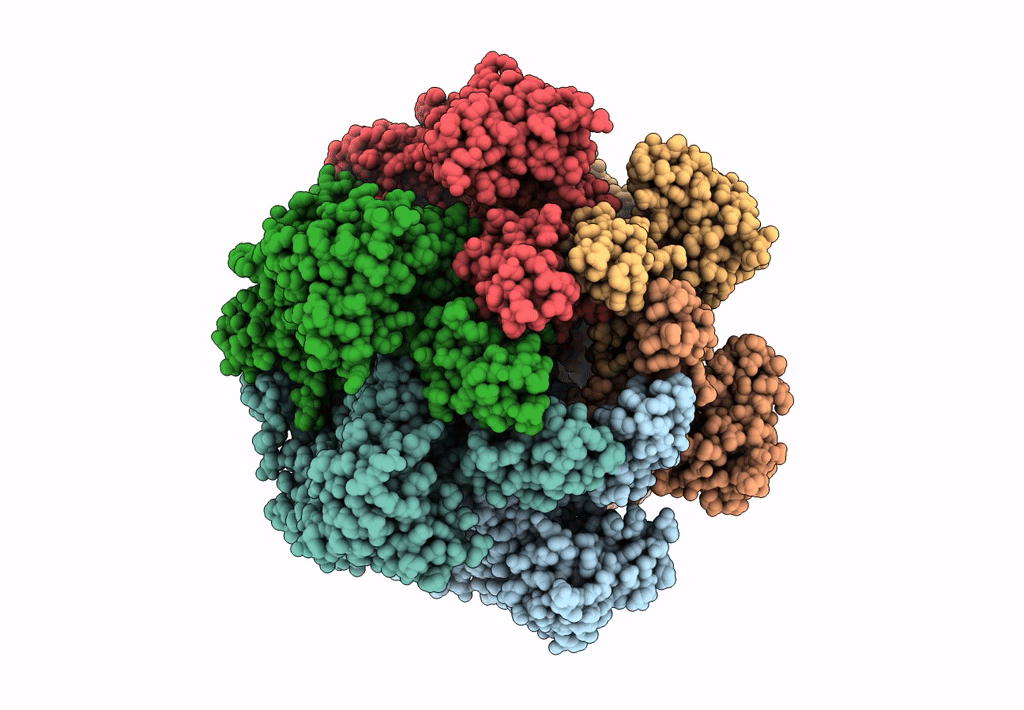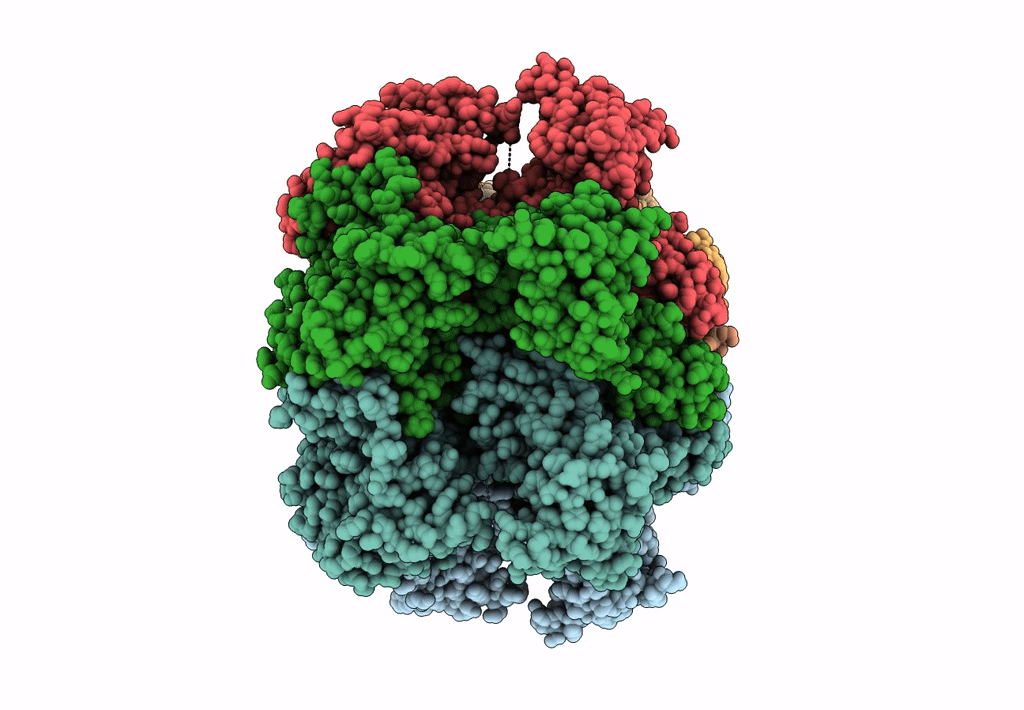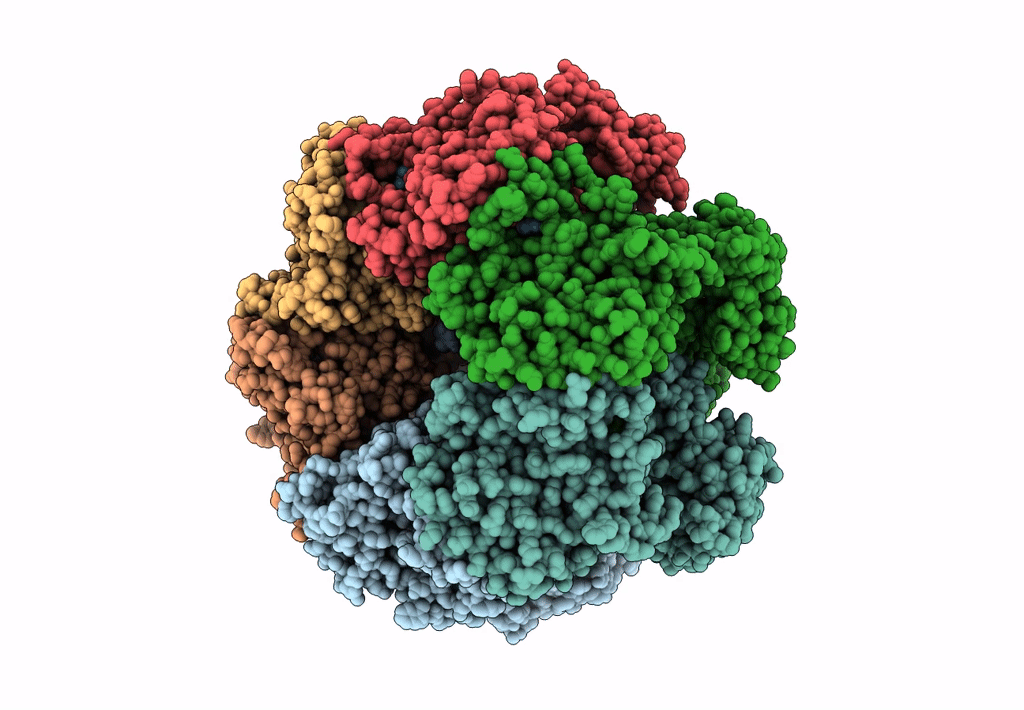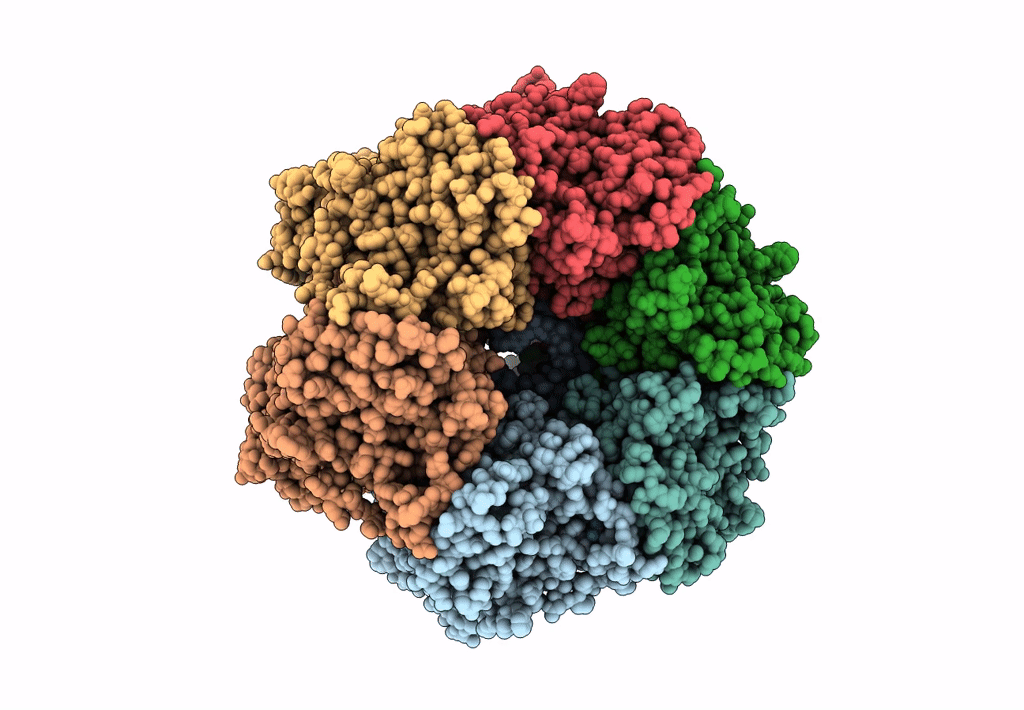
SsoMCM hexamer bound to Mg/ADP-BeFx and 46-mer DNA strand. Class 1
Biological Source:
Source Organism(s):
Saccharolobus solfataricus P2 (Taxon ID: 273057)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.45 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE