Deposition Date
2022-07-27
Release Date
2022-11-09
Last Version Date
2023-10-25
Entry Detail
PDB ID:
8DUG
Keywords:
Title:
Estrogen Receptor Alpha Ligand Binding Domain in Complex with (6'-hydroxy-1'-(4-(2-((<i>S</i>)-3-methylpyrrolidin-1-yl)ethoxy)phenyl)-1',4'-dihydro-2'<i>H</i>-spiro[cyclopropane-1,3'-isoquinolin]-2'-yl)(phenyl)methanone
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
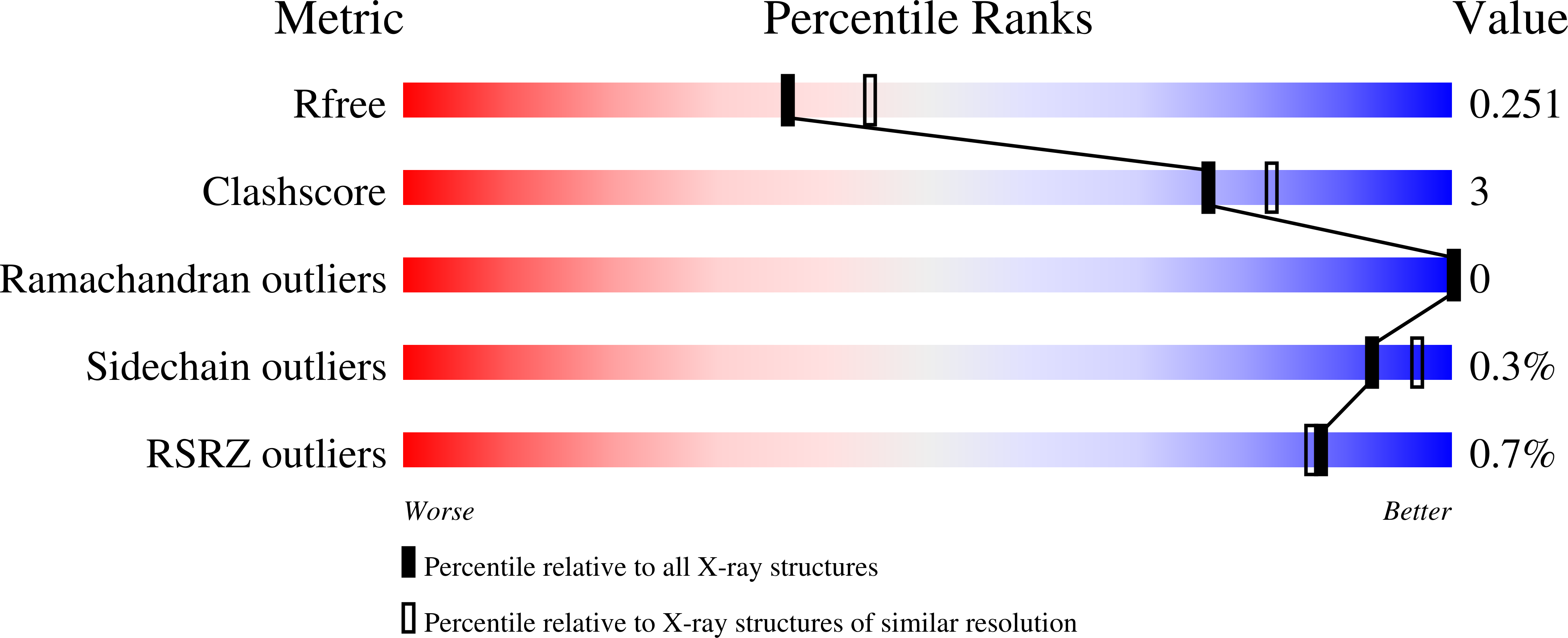
Resolution:
2.20 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 1 2 1