Deposition Date
2022-07-18
Release Date
2023-07-26
Last Version Date
2023-11-08
Entry Detail
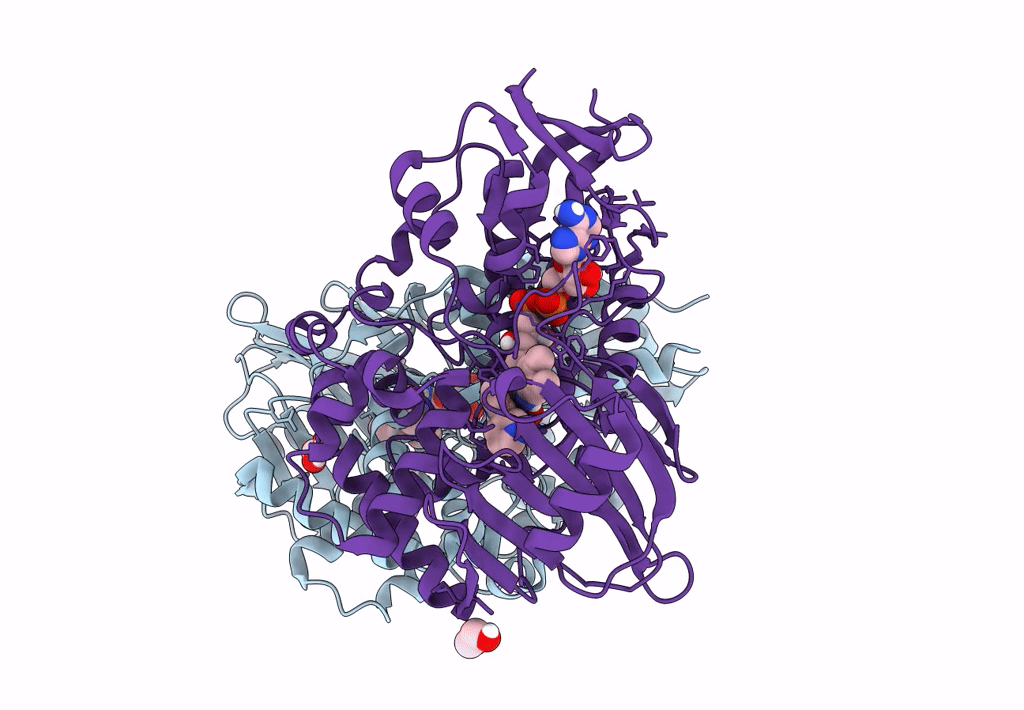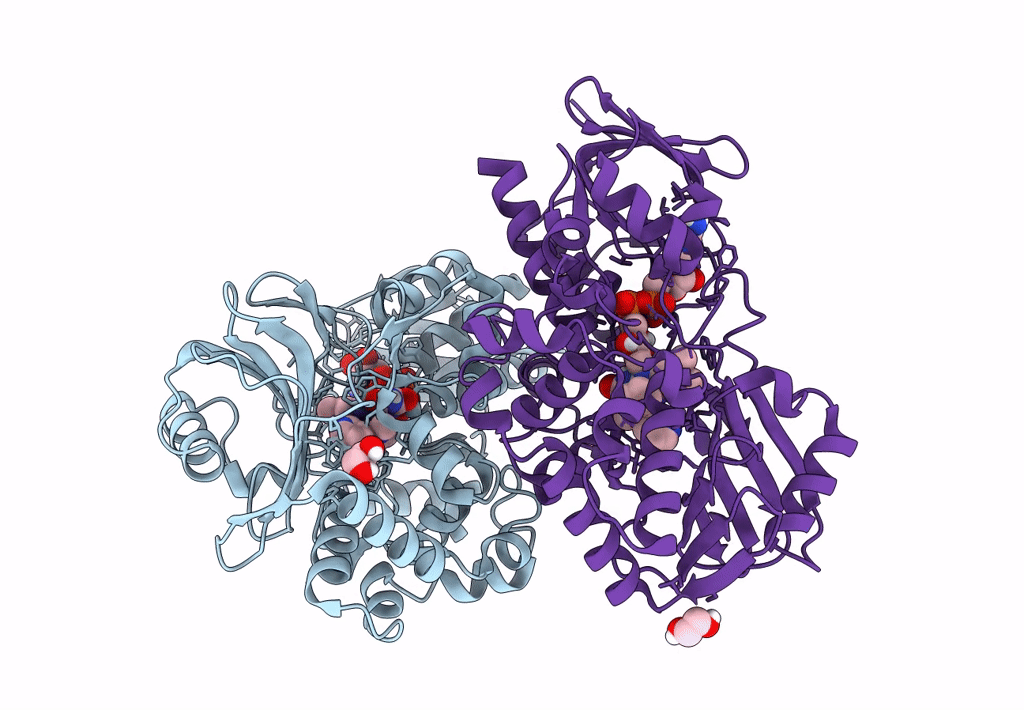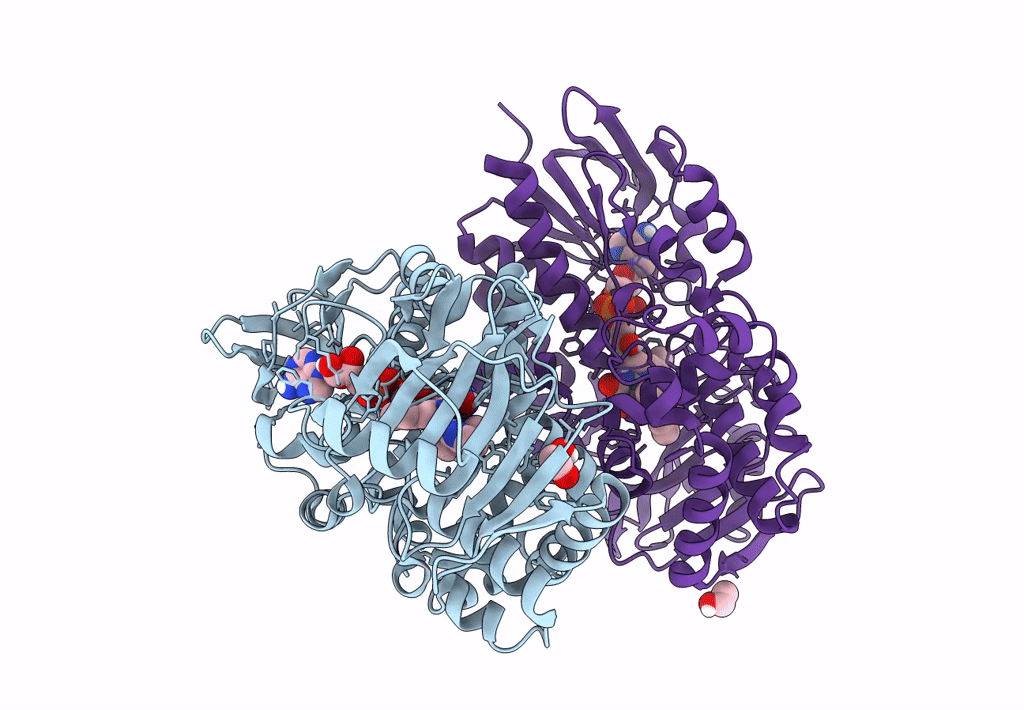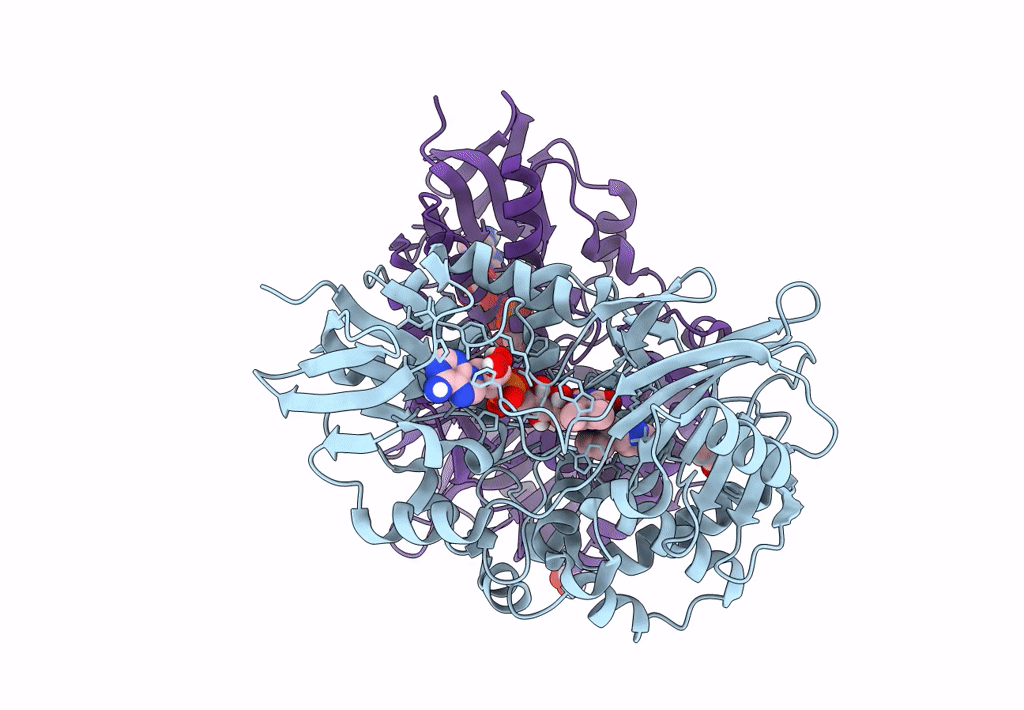
PDB ID:
8DQ8
Keywords:
Title:
The structure of NicA2 variant F104L/A107T/S146I/G317D/H368R/L449V/N462S in complex with N-methylmyosmine
Biological Source:
Source Organism(s):
Pseudomonas putida S16 (Taxon ID: 1042876)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.90 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 43


