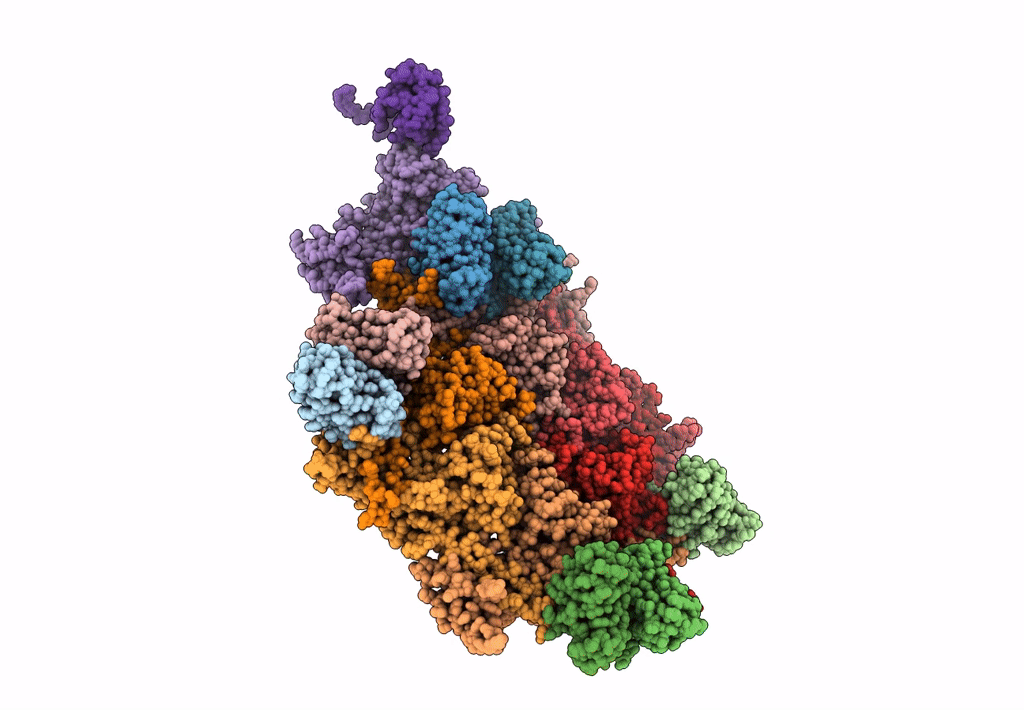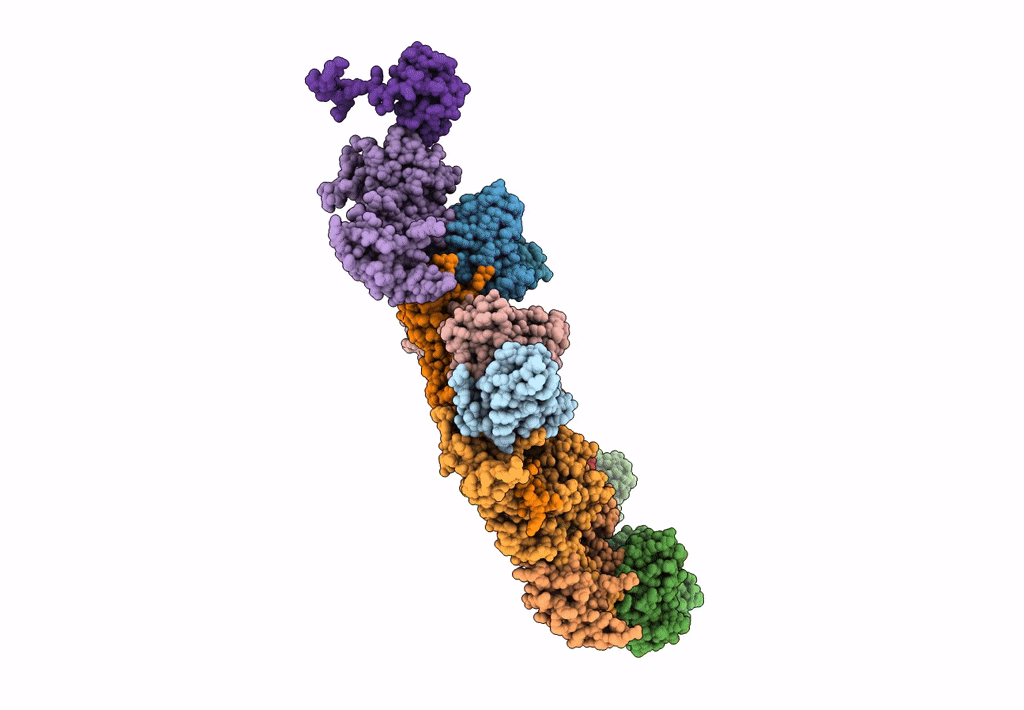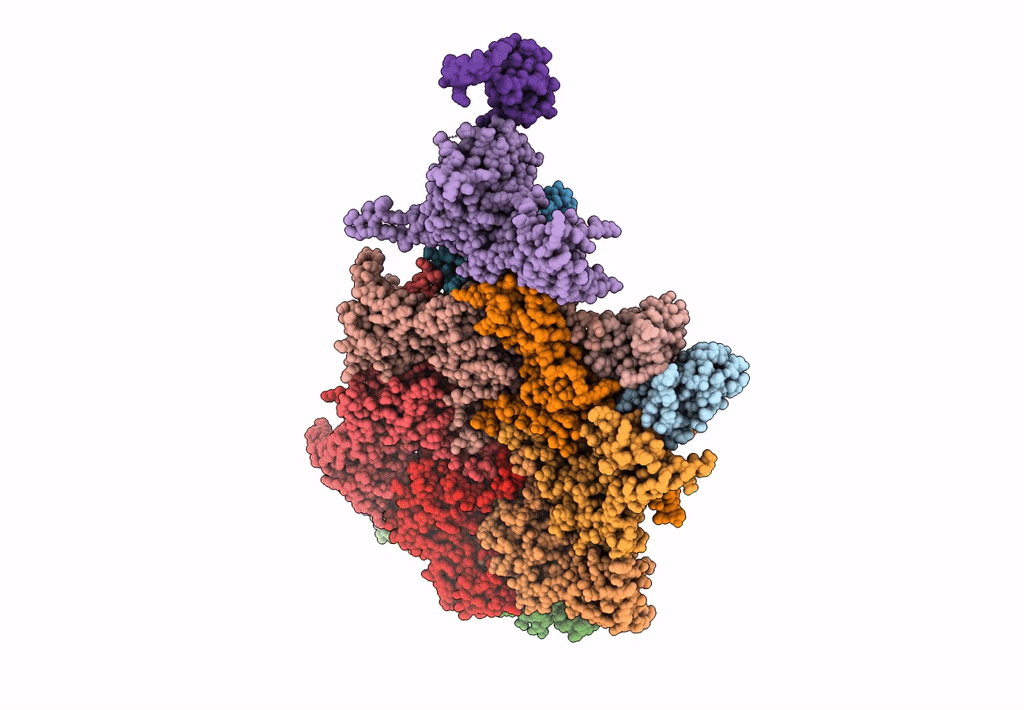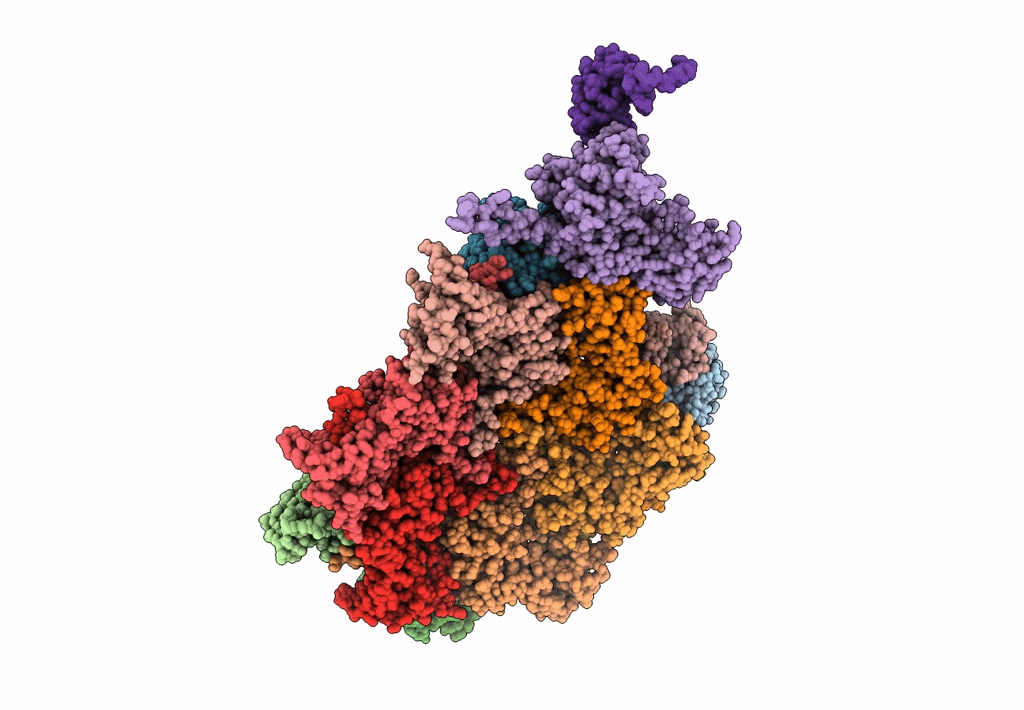
Abstact
Most of studied bacteriophages (phages) are terrestrial viruses. However, marine phages are shown to be highly involved in all levels of oceanic regulation. They are, however, still largely overlooked by the scientific community. By inducing cell lysis on half of the bacterial population daily, their role and influence on the bacterial biomass and evolution, as well as their impact in the global biogeochemical cycles, is undeniable. Cobetia marina virus 1 (Carin-1) is a member of the Podoviridae family infecting the γ-protoabacteria C. marina. Here, we present the almost complete, nearly-atomic resolution structure of Carin-1 comprising capsid, portal, and tail machineries at 3.5 Å, 3.8 Å and 3.9 Å, respectively, determined by cryo-electron microscopy (cryo-EM). Our experimental results, combined with AlphaFold2 (AF), allowed us to obtain the nearly-atomic structure of Carin-1 by fitting and refining the AF atomic models in the high resolution cryo-EM map, skipping the bottleneck of de-novo manual building and speeding up the structure determination process. Our structural results highlighted the T7-like nature of Carin1, as well as several novel structural features like the presence of short spikes on the capsid, reminiscent those described for Rhodobacter capsulatus gene transfer agent (RcGTA). This is, to our knowledge, the first time such assembly is described for a bacteriophage, shedding light into the common evolution and shared mechanisms between gene transfer agents and phages. This first full structure determined for a marine podophage allowed to propose an infection mechanism different than the one proposed for the archetypal podophage T7. IMPORTANCE Oceans play a central role in the carbon cycle on Earth and on the climate regulation (half of the planet's CO2 is absorbed by phytoplankton photosynthesis in the oceans and just as much O2 is liberated). The understanding of the biochemical equilibriums of marine biology represents a major goal for our future. By lysing half of the bacterial population every day, marine bacteriophages are key actors of these equilibriums. Despite their importance, these marine phages have, so far, only been studied a little and, in particular, structural insights are currently lacking, even though they are fundamental for the understanding of the molecular mechanisms of their mode of infection. The structures described in our manuscript allow us to propose an infection mechanism that differs from the one proposed for the terrestrial T7 virus, and might also allow us to, in the future, better understand the way bacteriophages shape the global ecosystem.