Deposition Date
2023-02-01
Release Date
2023-12-20
Last Version Date
2024-11-13
Entry Detail
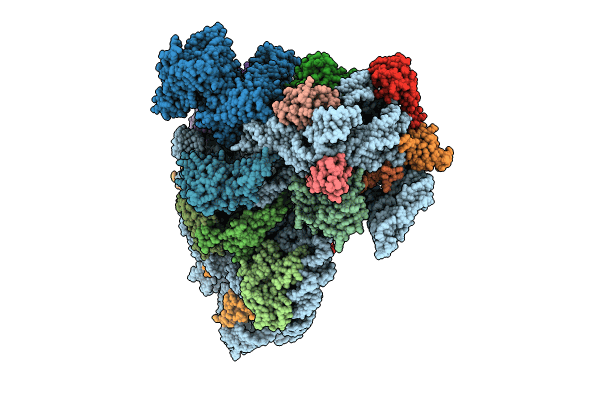
PDB ID:
8CED
Keywords:
Title:
Rnase R bound to a 30S degradation intermediate (State I - head-turning)
Biological Source:
Source Organism(s):
Bacillus subtilis subsp. subtilis str. 168 (Taxon ID: 224308)
Method Details:
Experimental Method:
Resolution:
4.15 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


