Deposition Date
2023-01-17
Release Date
2023-07-19
Last Version Date
2023-11-15
Entry Detail
PDB ID:
8C7S
Keywords:
Title:
Transcriptional pleiotropic repressor CodY from Staphylococcus aureus in complex with Ile, GTP, and a 30-bp DNA fragment encompassing two overlapping binding sites
Biological Source:
Source Organism(s):
Staphylococcus aureus (strain USA300) (Taxon ID: 367830)
Staphylococcus aureus (Taxon ID: 1280)
Staphylococcus aureus (Taxon ID: 1280)
Expression System(s):
Method Details:
Experimental Method:
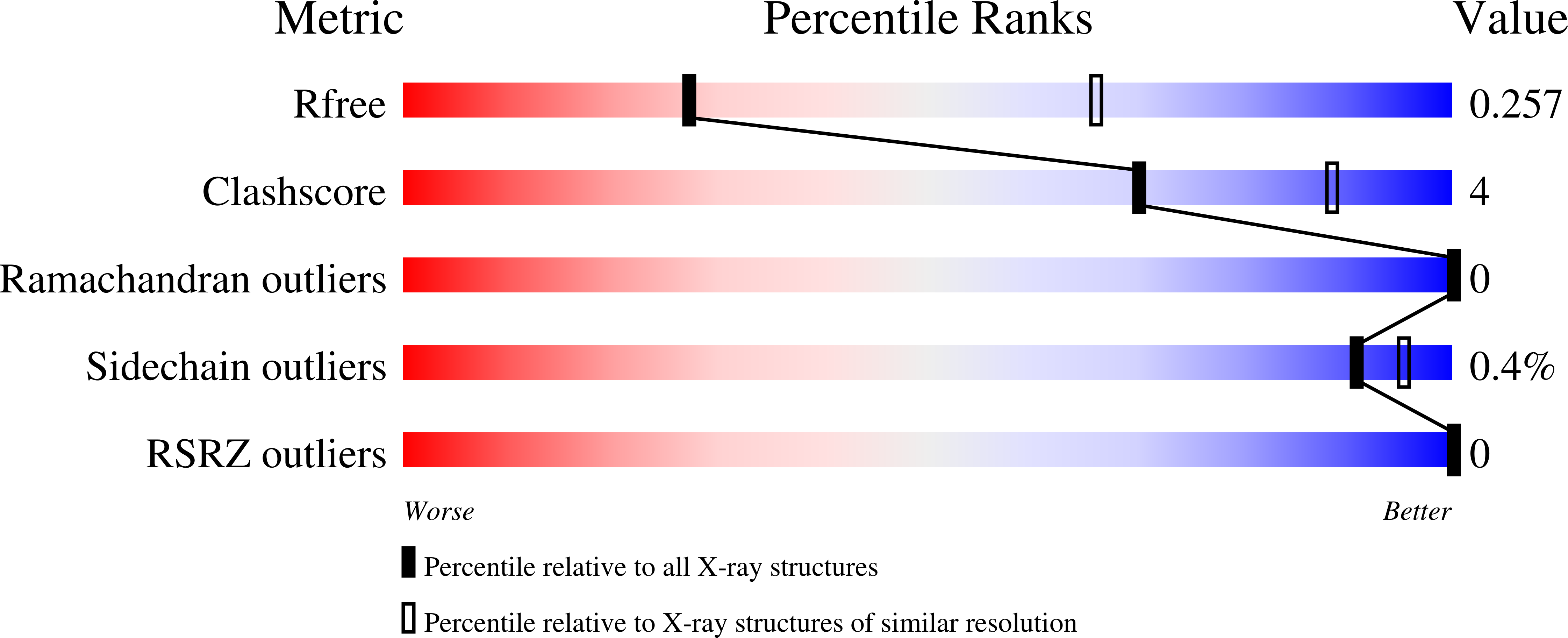
Resolution:
3.05 Å
R-Value Free:
0.25
R-Value Work:
0.21
Space Group:
P 61 2 2