Deposition Date
2022-11-21
Release Date
2024-03-13
Last Version Date
2024-11-06
Entry Detail
PDB ID:
8BQU
Keywords:
Title:
Molecular basis of ZP3/ZP1 heteropolymerization: crystal structure of a native vertebrate egg coat filament
Biological Source:
Source Organism:
Oryzias latipes (Taxon ID: 8090)
Method Details:
Experimental Method:
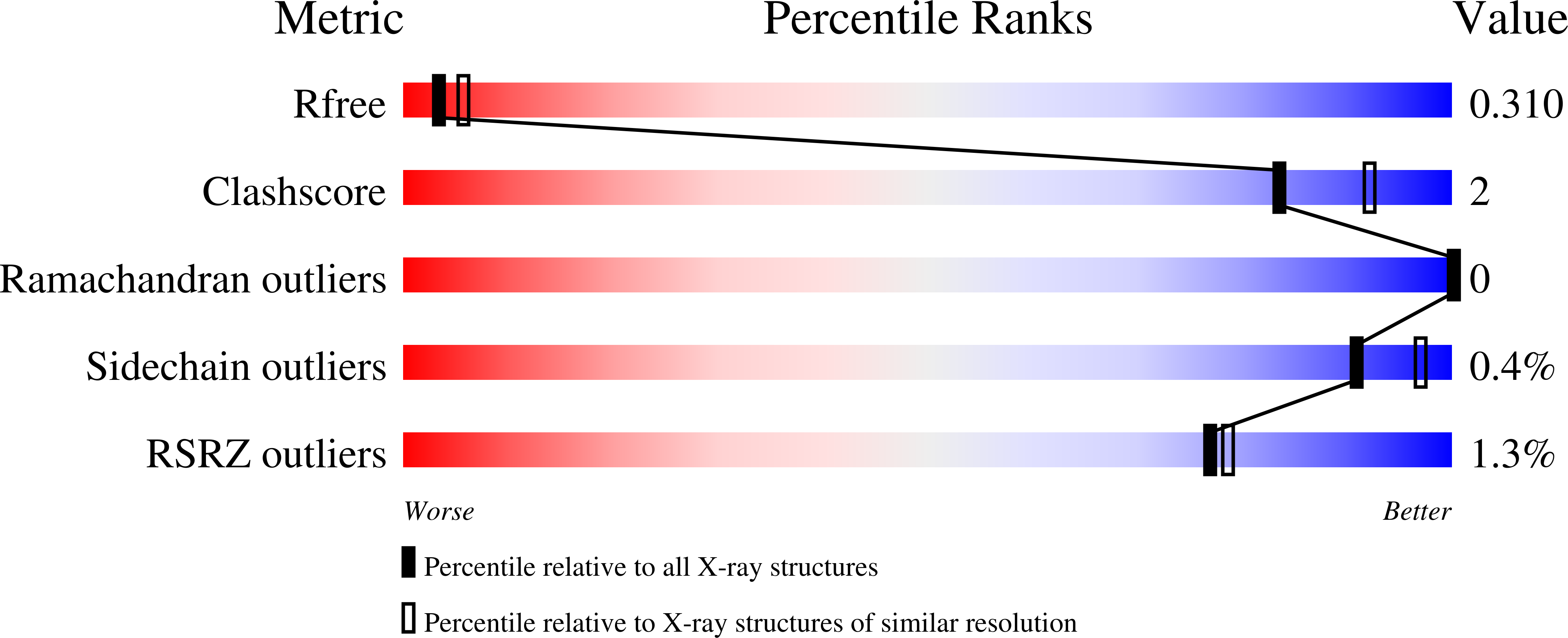
Resolution:
2.70 Å
R-Value Free:
0.30
R-Value Work:
0.24
R-Value Observed:
0.25
Space Group:
P 32 2 1