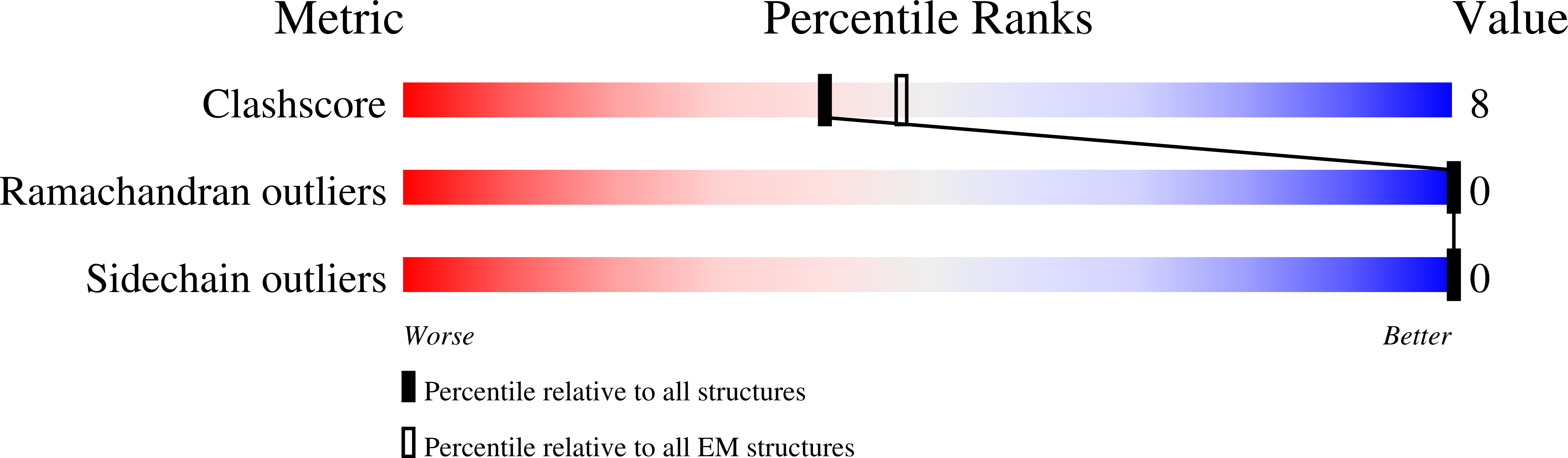
Deposition Date
2022-11-10
Release Date
2023-08-02
Last Version Date
2024-07-24
Entry Detail
PDB ID:
8BMR
Keywords:
Title:
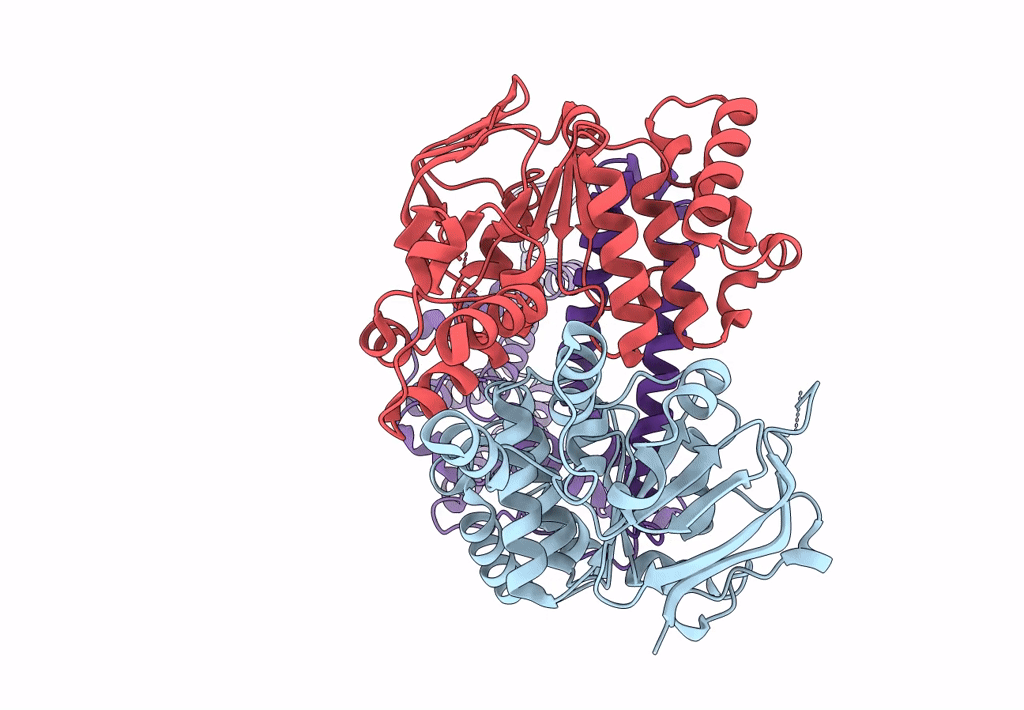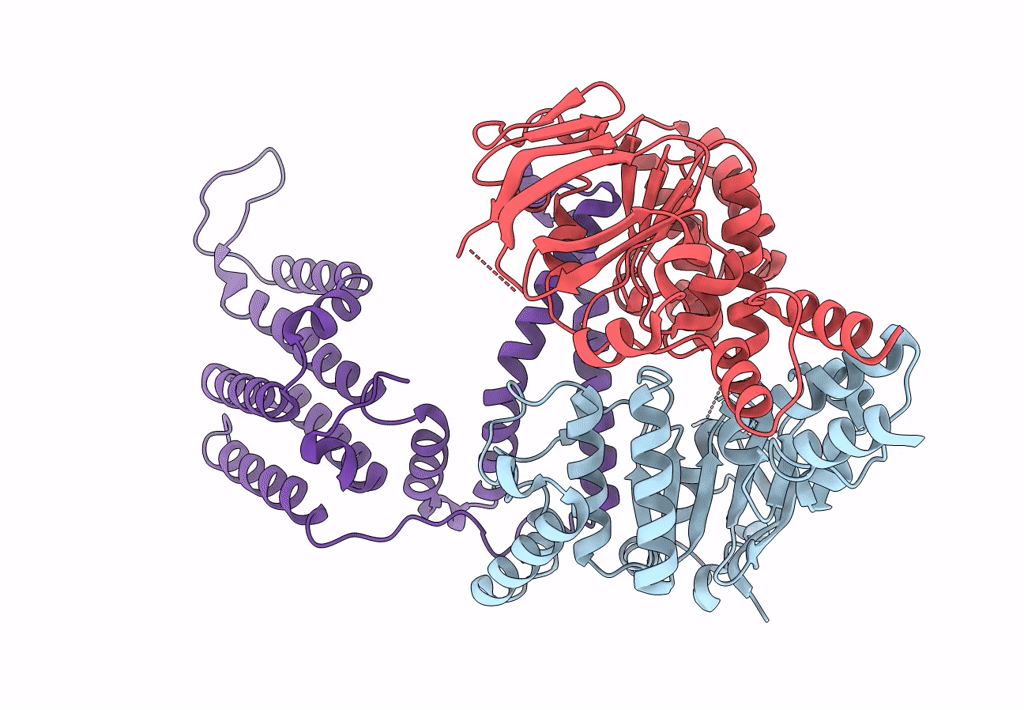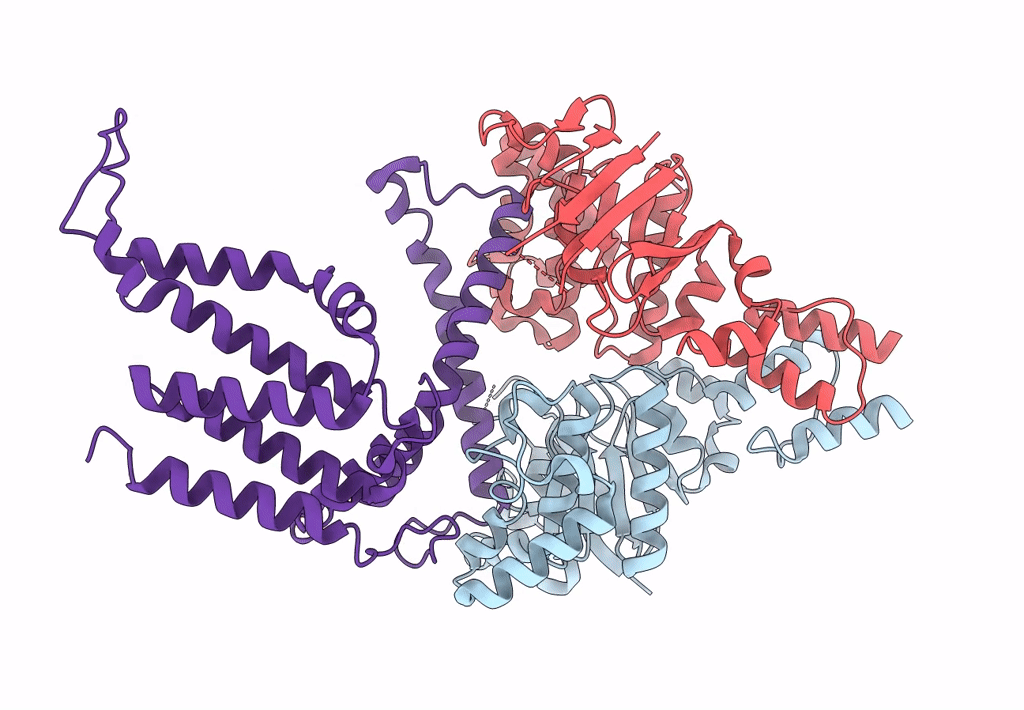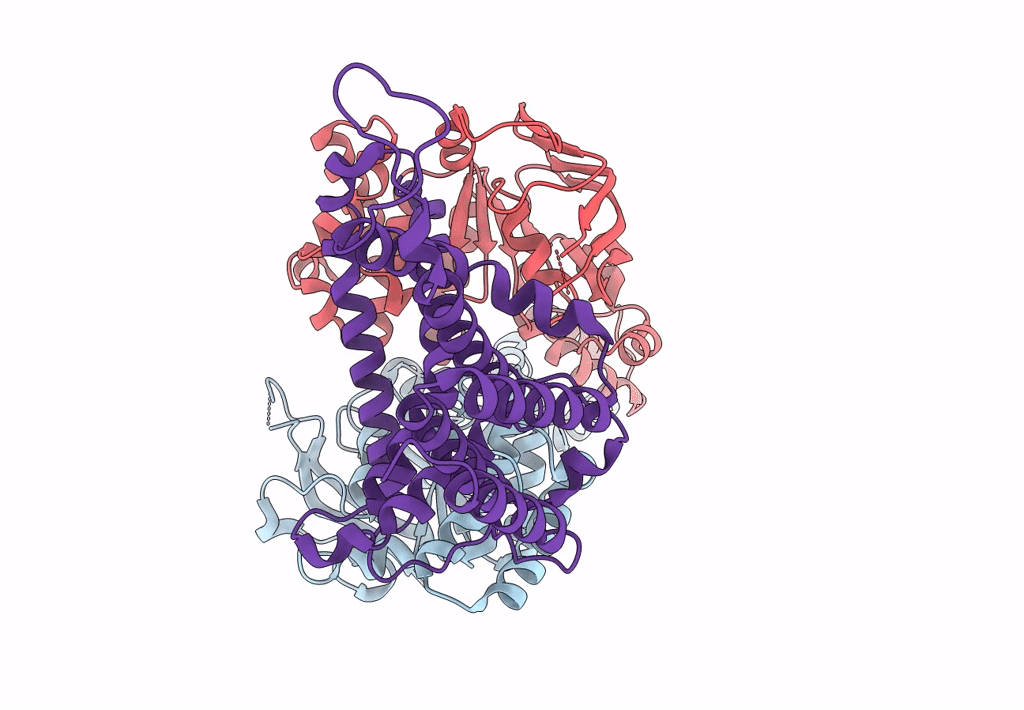
Cryo-EM structure of the wild-type solitary ECF module in MSP2N2 lipid nanodiscs in the ATPase open and nucleotide-free conformation
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE