Deposition Date
2022-10-21
Release Date
2023-01-11
Last Version Date
2024-10-23
Entry Detail
PDB ID:
8BEH
Keywords:
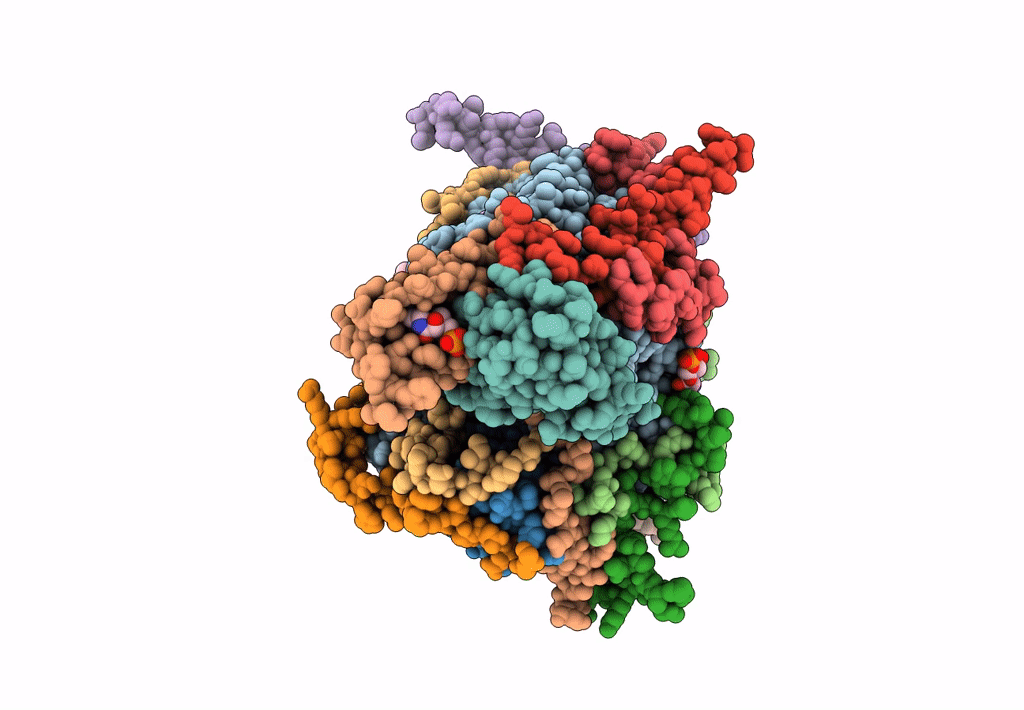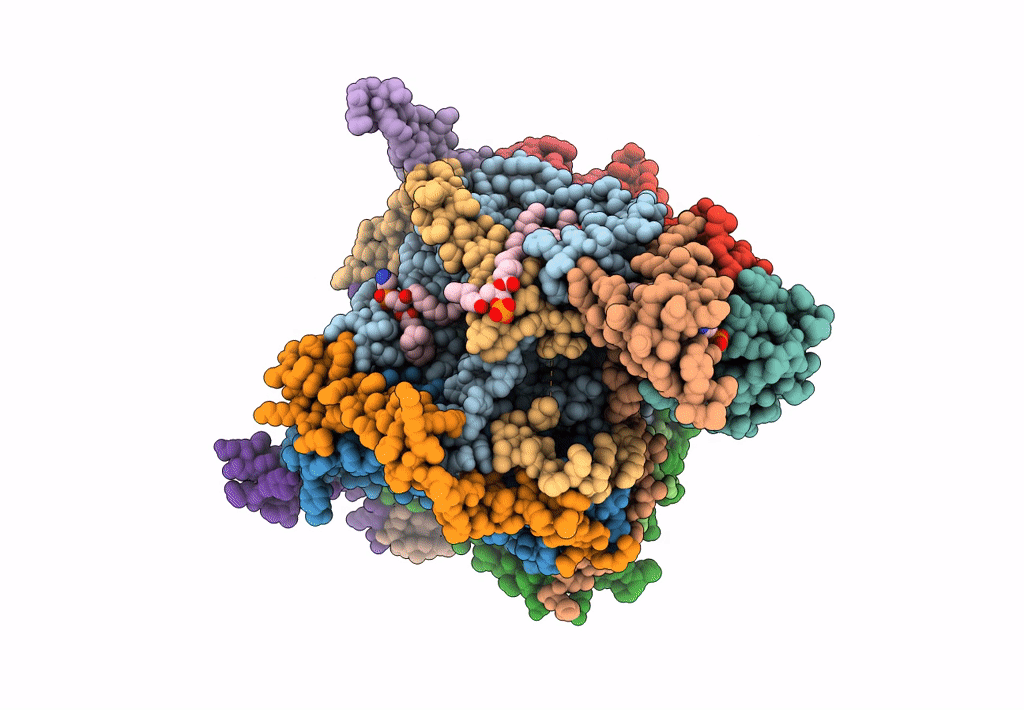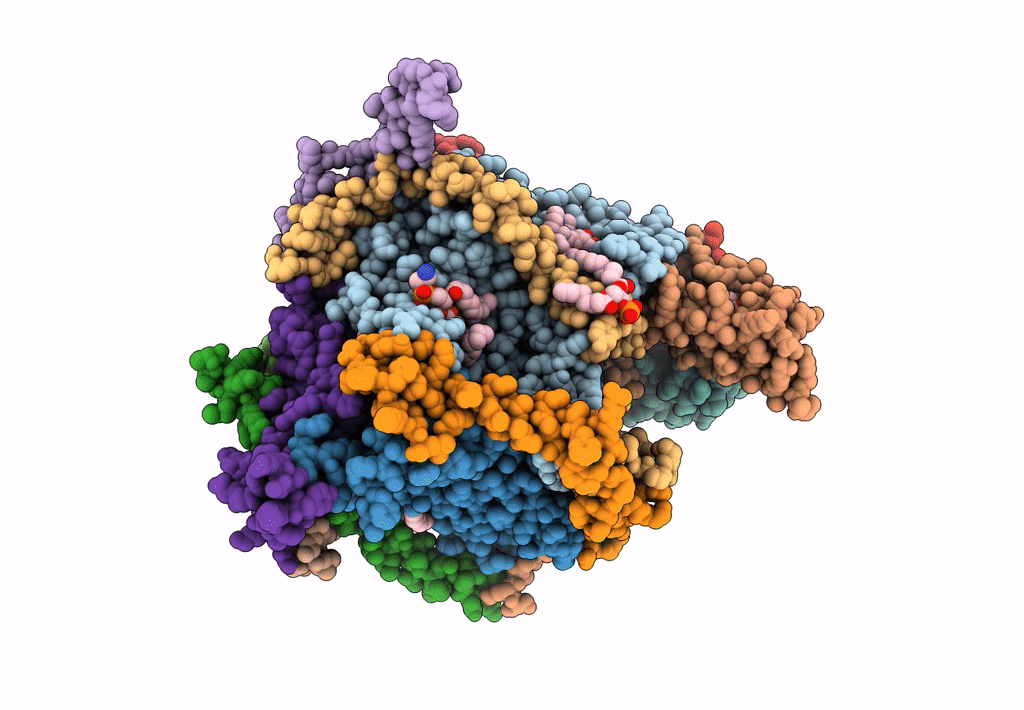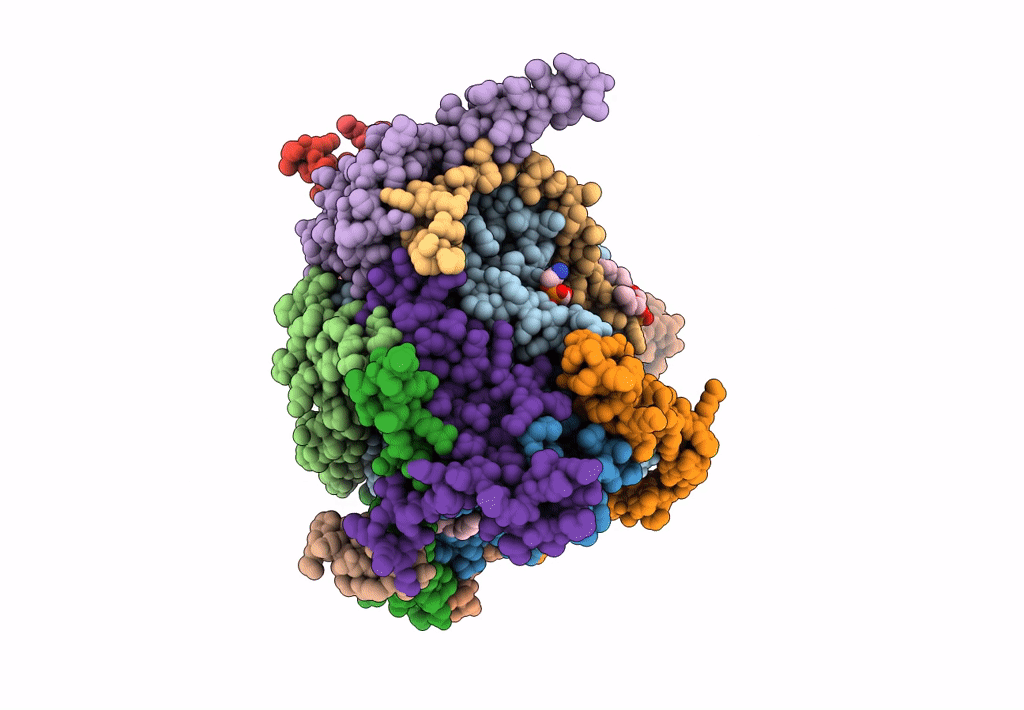
Title:
Cryo-EM structure of the Arabidopsis thaliana I+III2 supercomplex (CI membrane tip)
Biological Source:
Source Organism(s):
Arabidopsis thaliana (Taxon ID: 3702)
Method Details:
Experimental Method:
Resolution:
2.29 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


