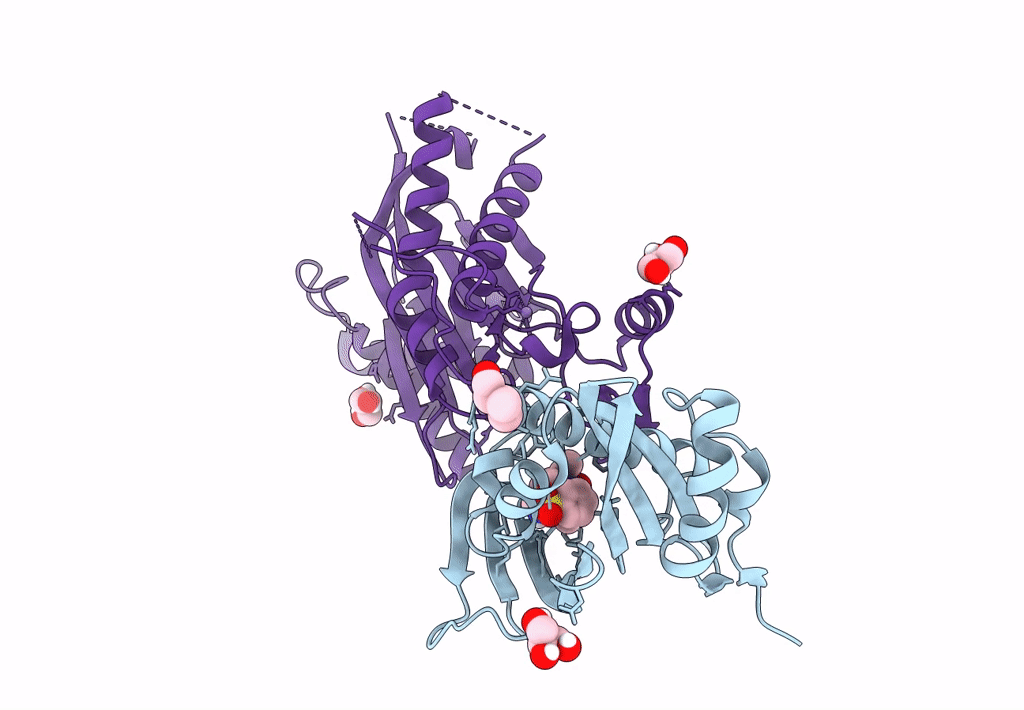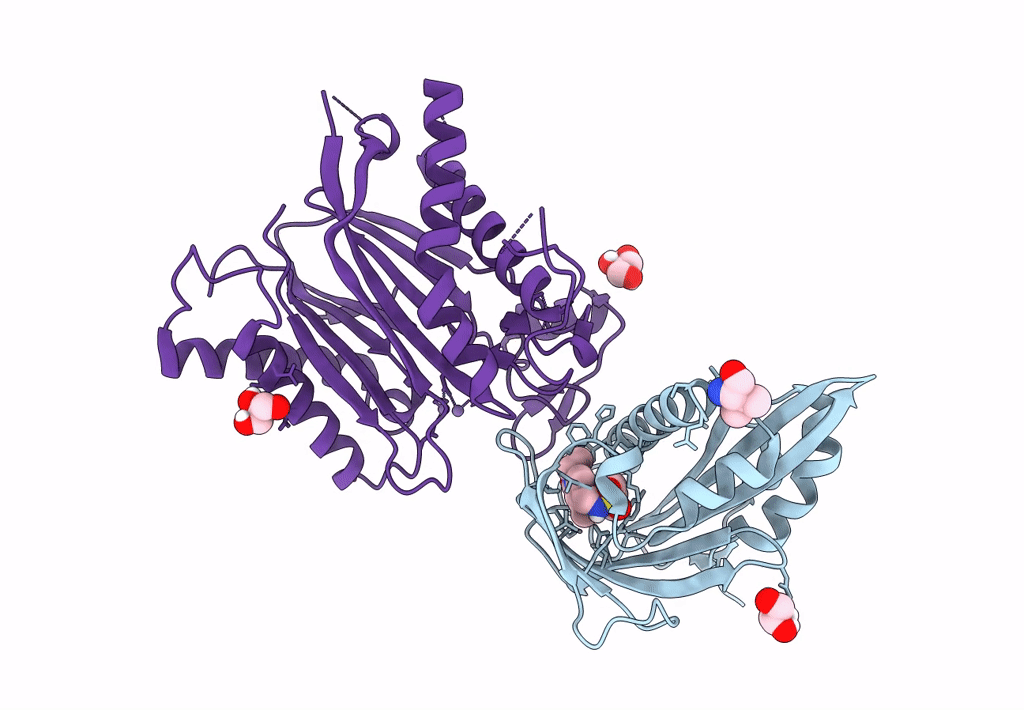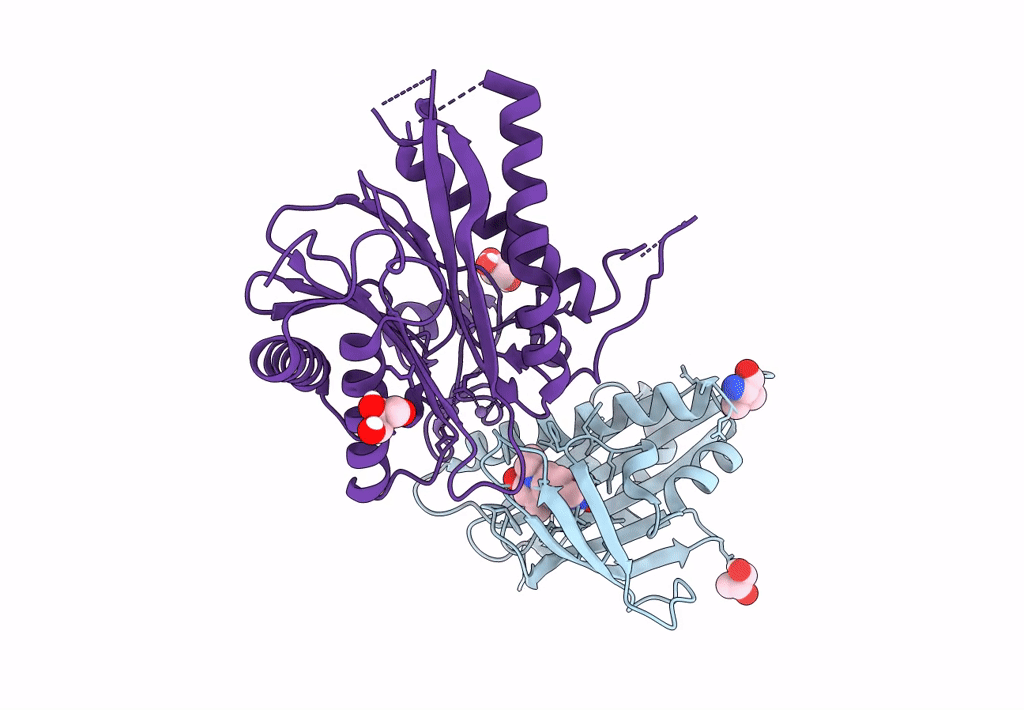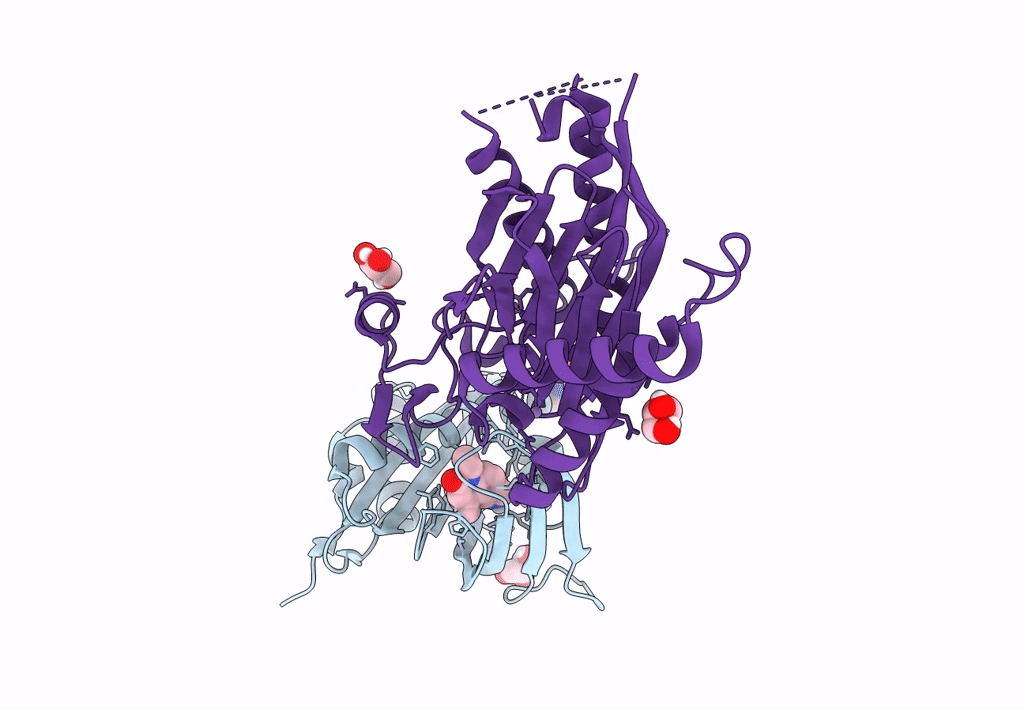
Deposition Date
2022-09-02
Release Date
2023-03-22
Last Version Date
2024-02-28
Entry Detail
PDB ID:
8AY8
Keywords:
Title:
X-RAY CRYSTAL STRUCTURE OF THE CsPYL1(V112L, T135L,F137I, T153I, V168A)-iSB9-HAB1 TERNARY COMPLEX
Biological Source:
Source Organism(s):
Citrus sinensis (Taxon ID: 2711)
Arabidopsis thaliana (Taxon ID: 3702)
Arabidopsis thaliana (Taxon ID: 3702)
Expression System(s):
Method Details:
Experimental Method:
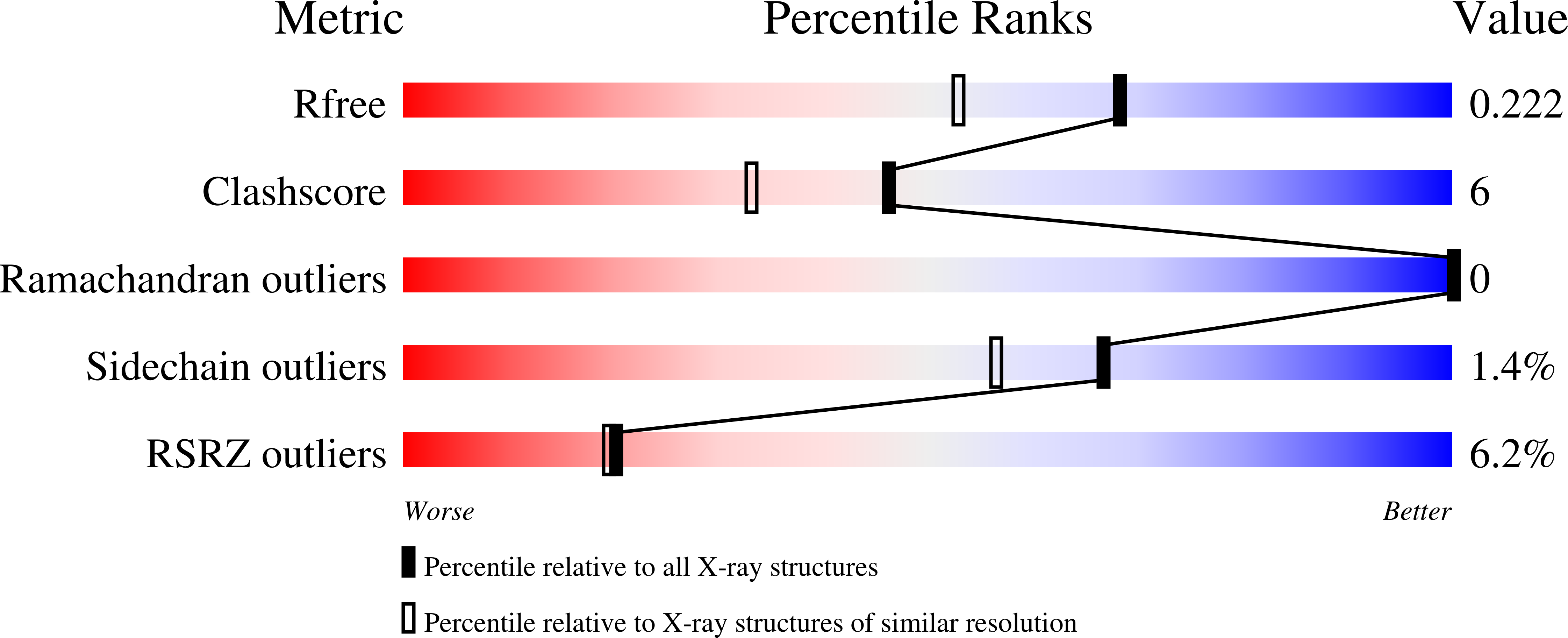
Resolution:
1.78 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21