Deposition Date
2022-08-19
Release Date
2023-03-15
Last Version Date
2025-10-01
Entry Detail
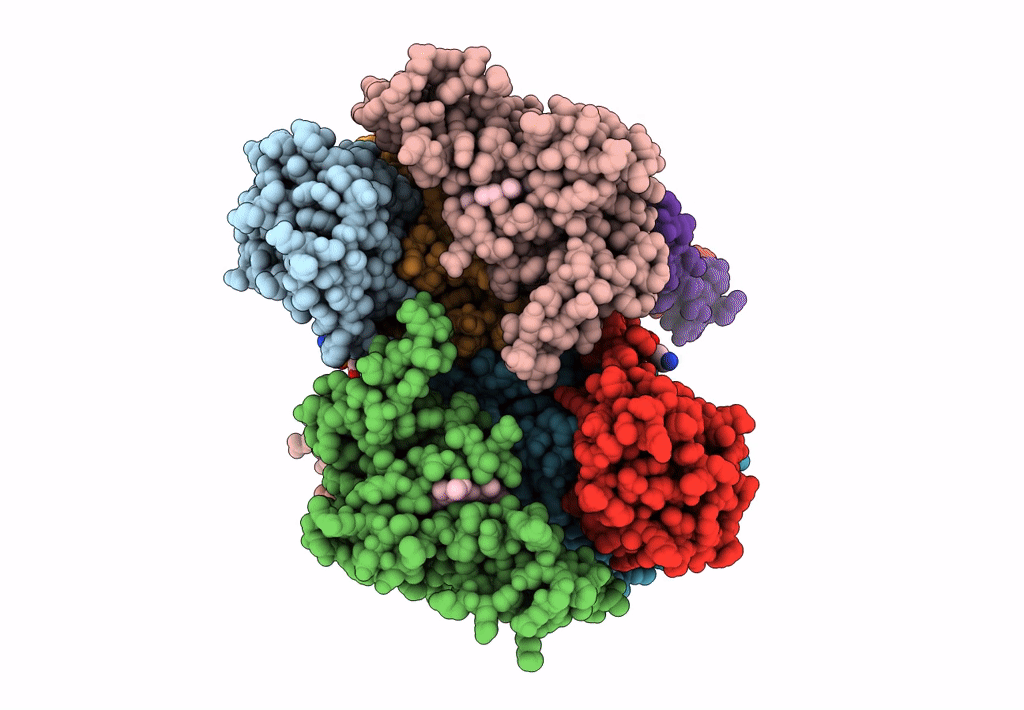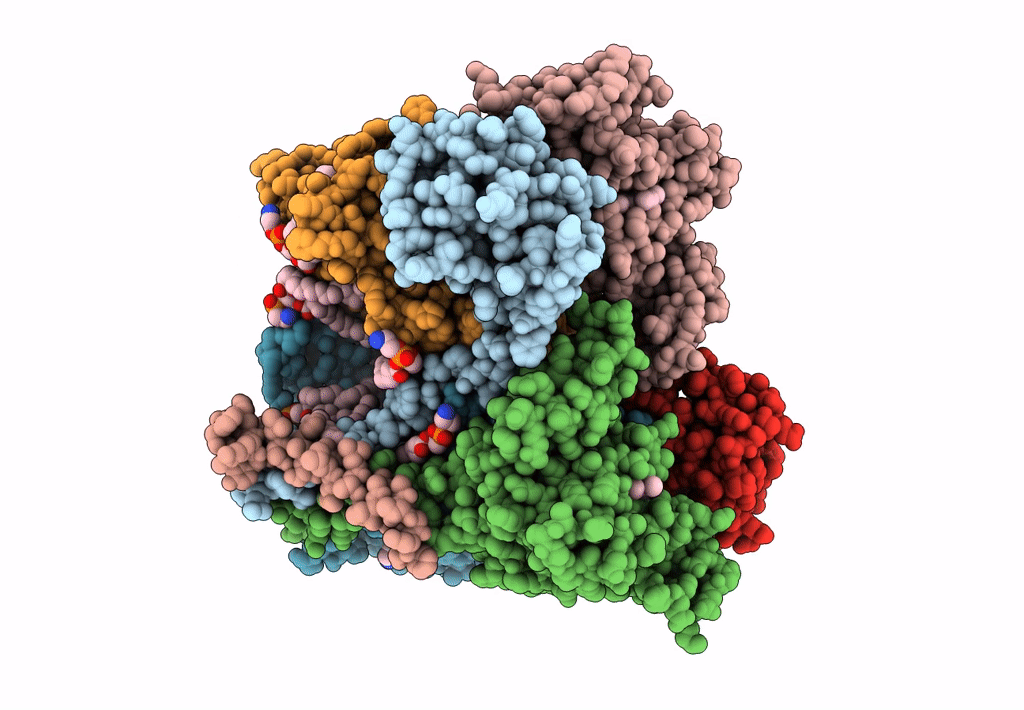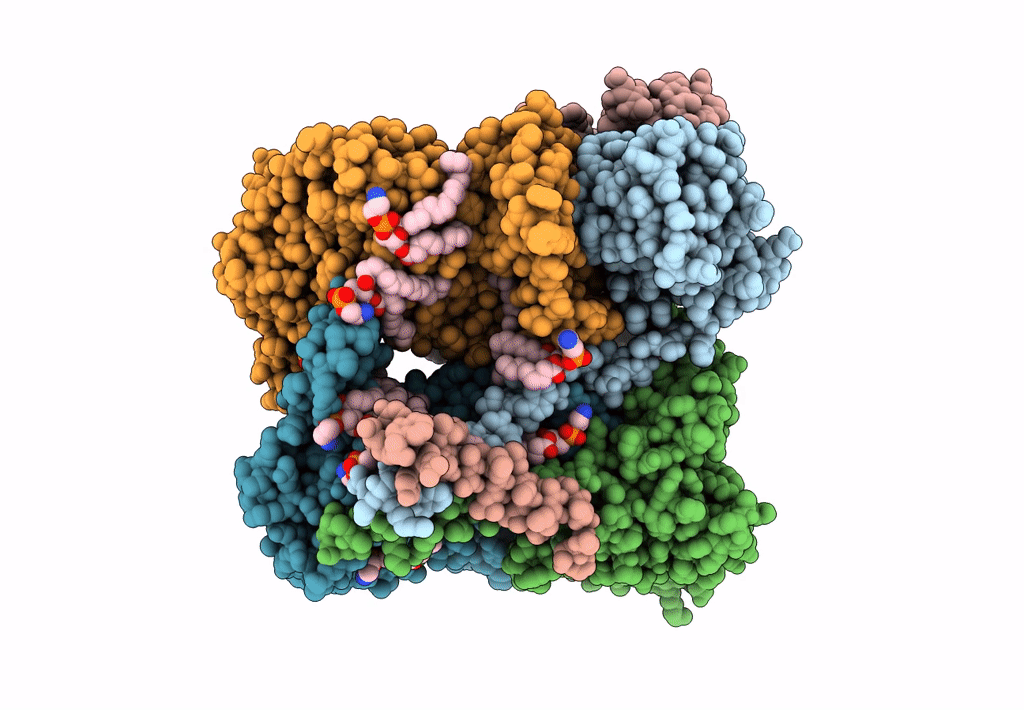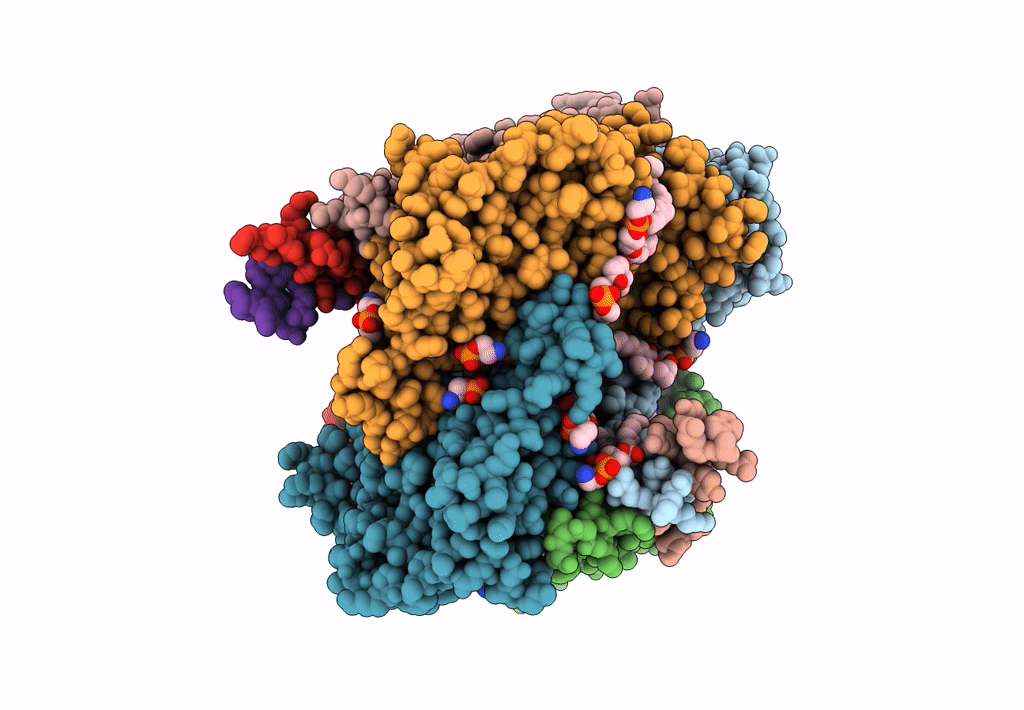
PDB ID:
8ASI
Keywords:
Title:
Four subunit cytochrome b-c1 complex from Rhodobacter sphaeroides in native nanodiscs - consensus refinement in the b-b conformation
Biological Source:
Source Organism(s):
Cereibacter sphaeroides 2.4.1 (Taxon ID: 272943)
Method Details:
Experimental Method:
Resolution:
2.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


