Deposition Date
2022-07-18
Release Date
2023-04-05
Last Version Date
2024-10-23
Entry Detail
PDB ID:
8AFV
Keywords:
Title:
DaArgC3 - Engineered Formyl Phosphate Reductase with 3 substitutions (S178V, G182V, L233I)
Biological Source:
Source Organism(s):
Denitrovibrio acetiphilus DSM 12809 (Taxon ID: 522772)
Expression System(s):
Method Details:
Experimental Method:
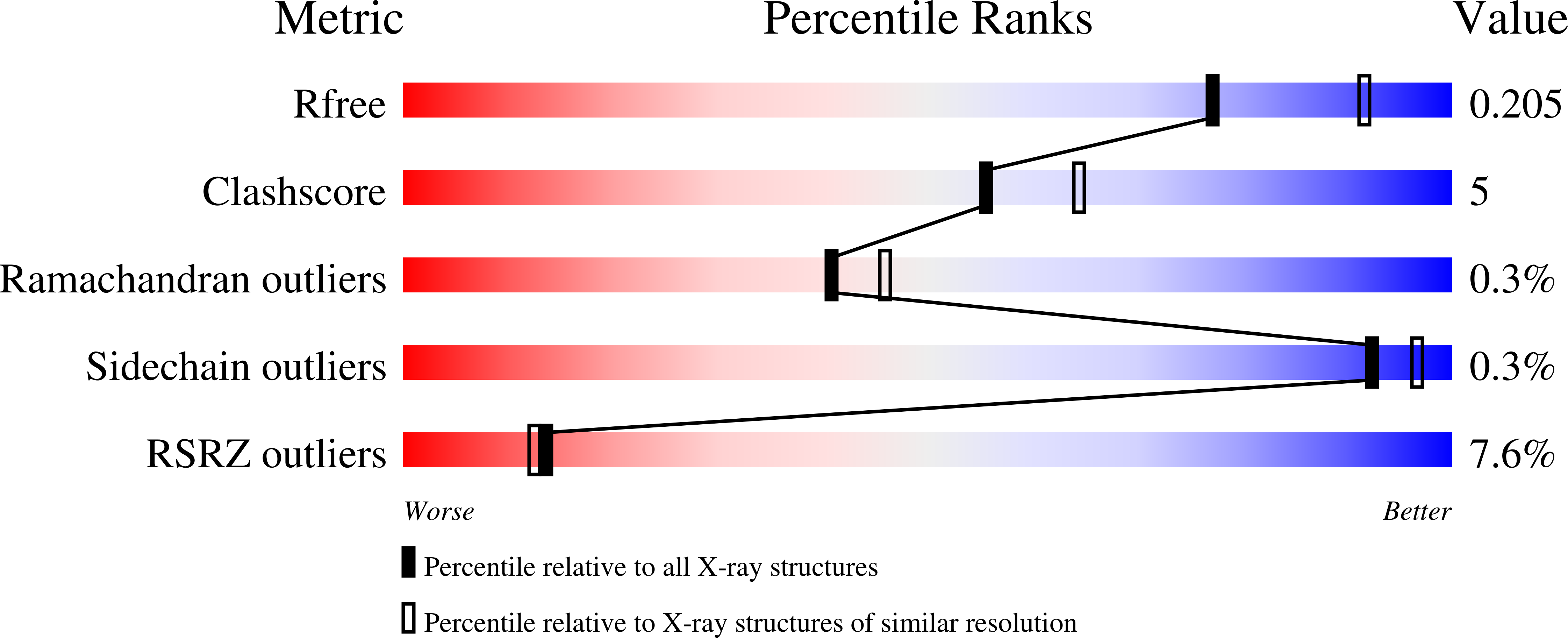
Resolution:
2.19 Å
R-Value Free:
0.20
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21