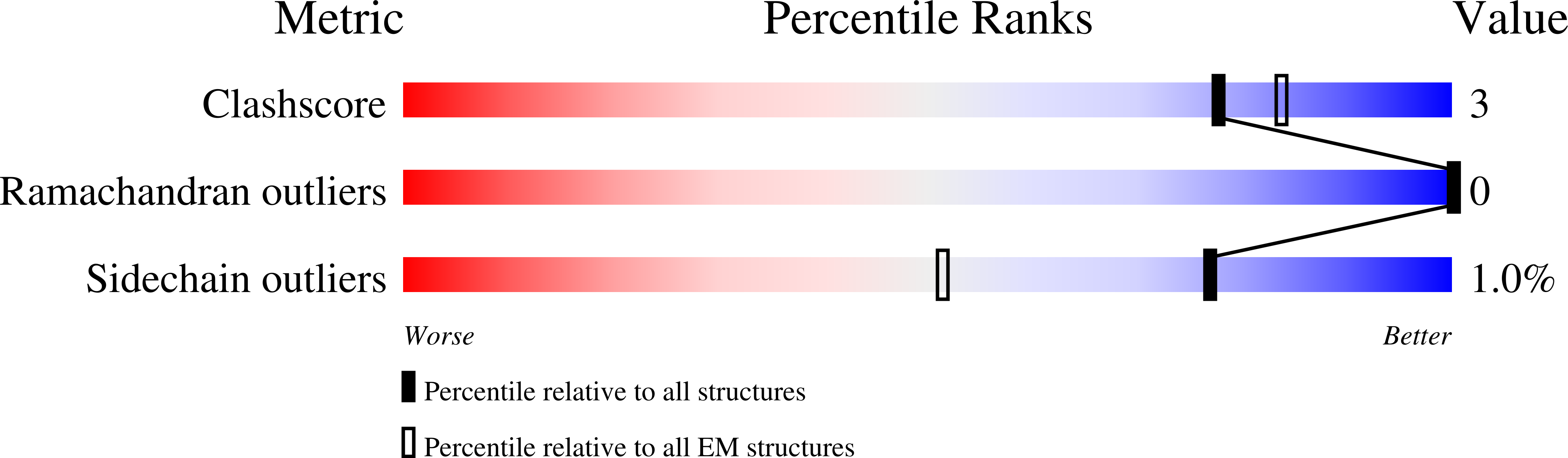
Deposition Date
2022-06-02
Release Date
2023-06-14
Last Version Date
2024-11-20
Entry Detail
PDB ID:
8A1W
Keywords:
Title:
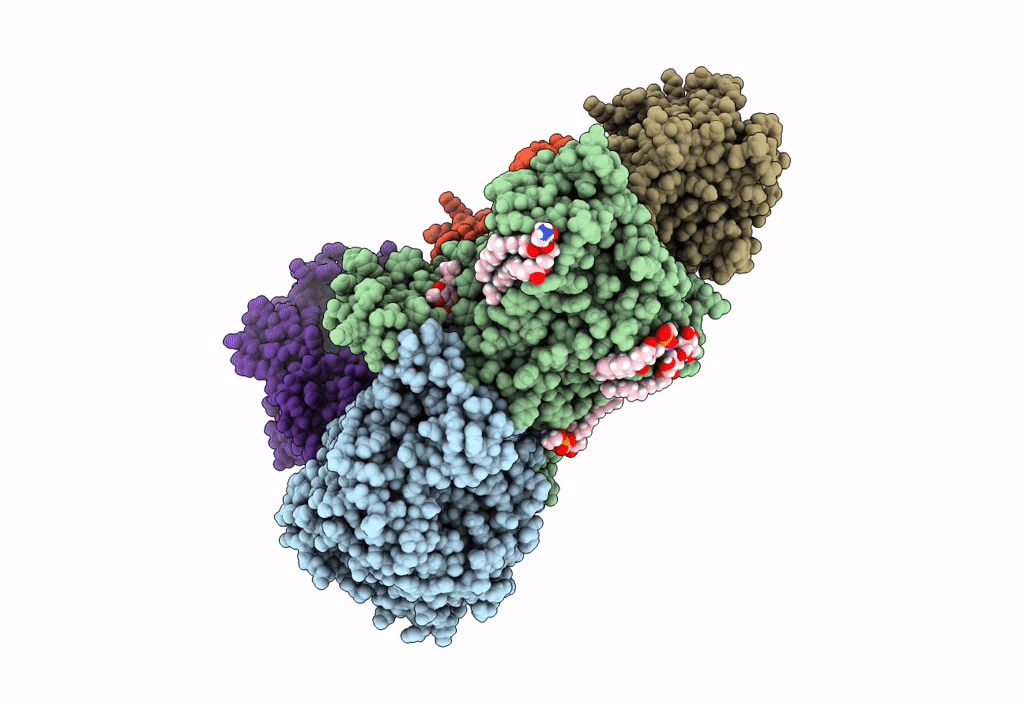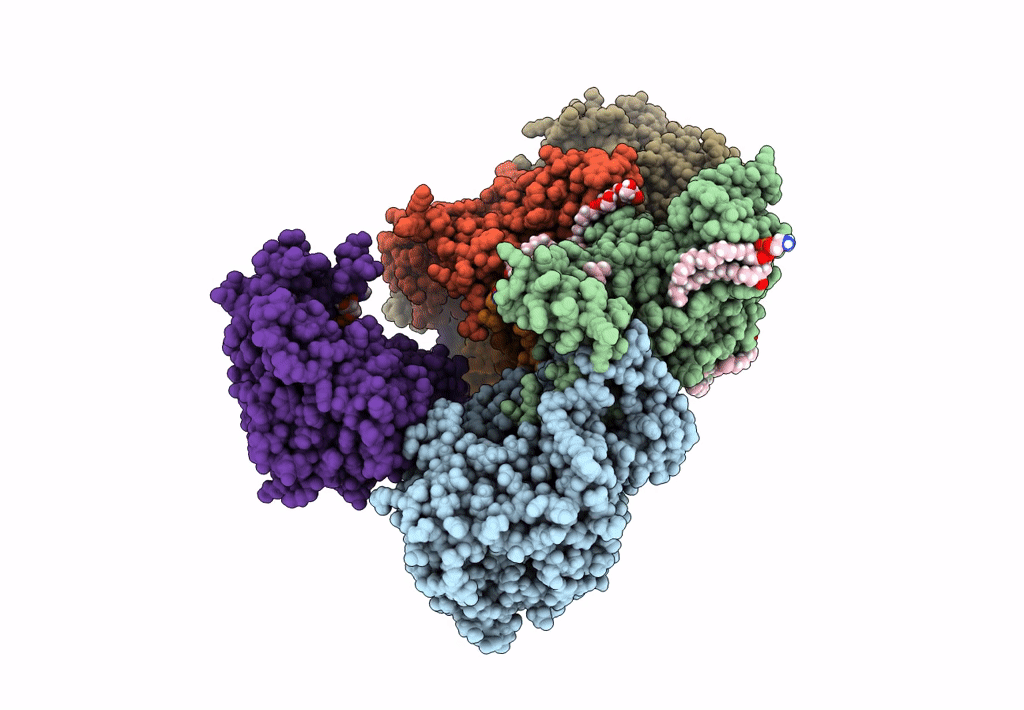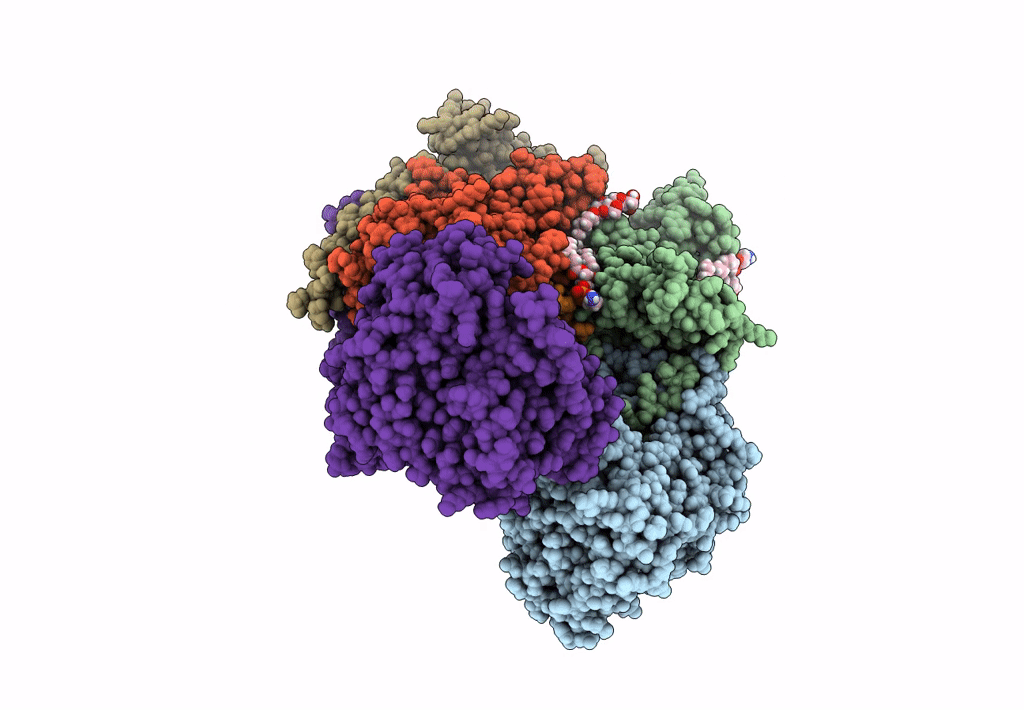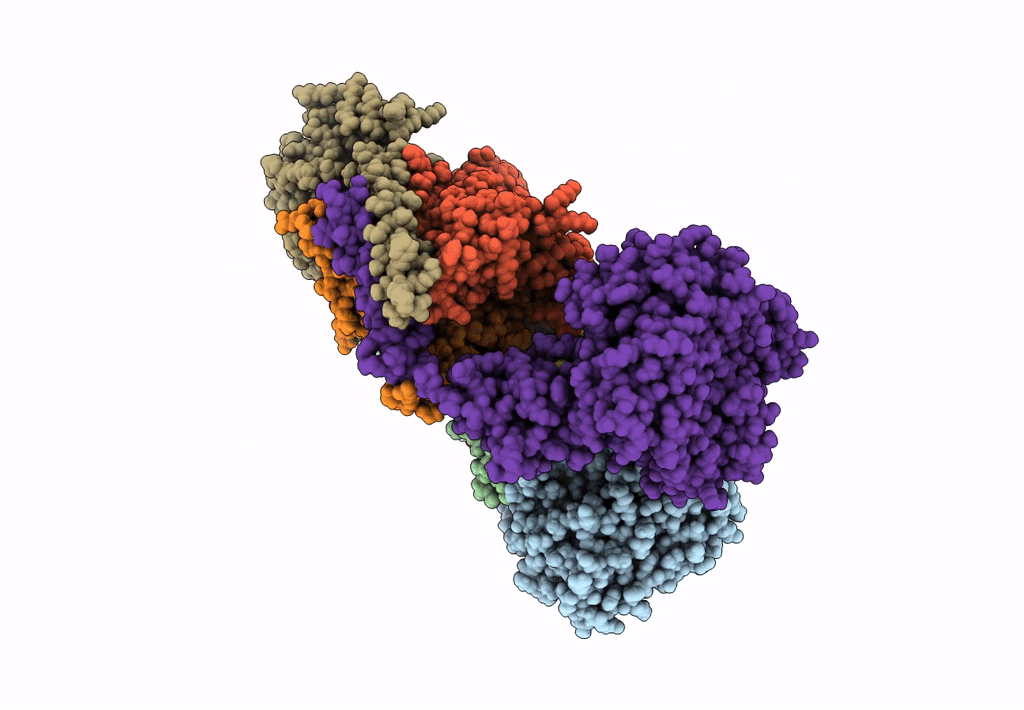
Sodium pumping NADH-quinone oxidoreductase with substrate Q1
Biological Source:
Source Organism:
Vibrio cholerae (Taxon ID: 666)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.56 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE