Deposition Date
2022-05-26
Release Date
2023-01-18
Last Version Date
2024-11-13
Entry Detail
PDB ID:
8A00
Keywords:
Title:
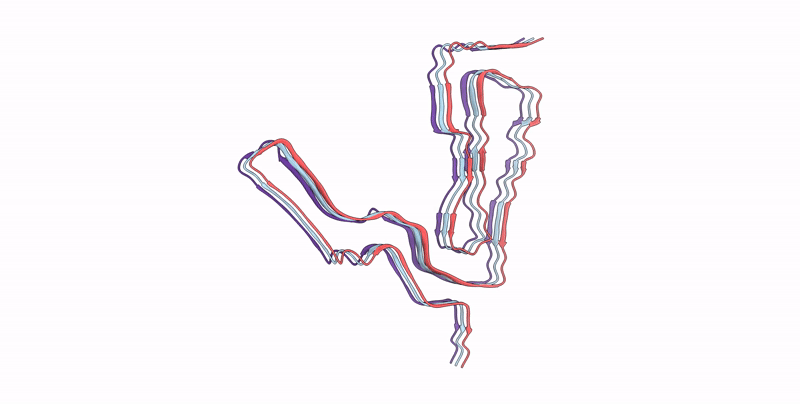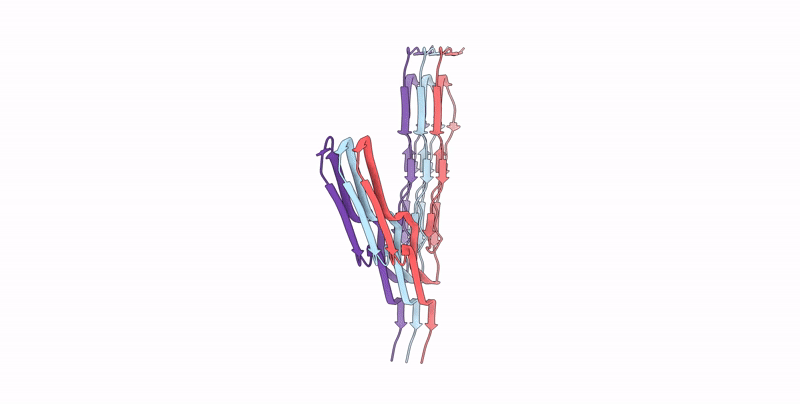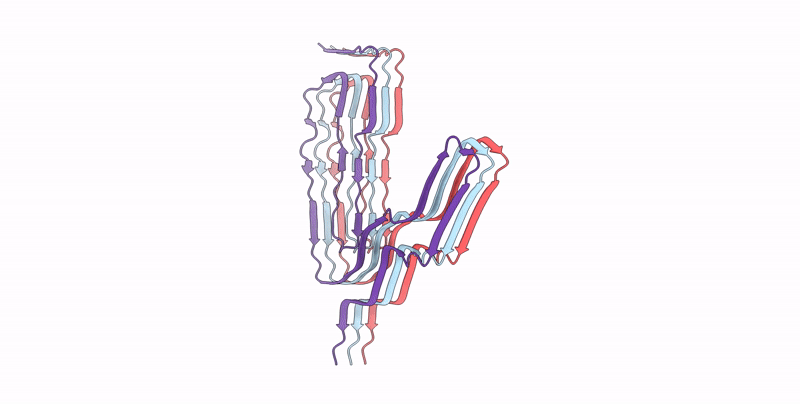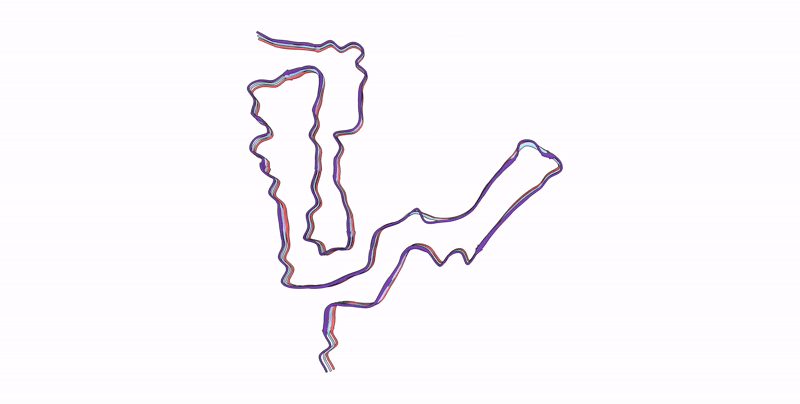
Infectious mouse-adapted ME7 scrapie prion fibril purified from terminally-infected mouse brains
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Method Details:
Experimental Method:
Resolution:
2.60 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL


